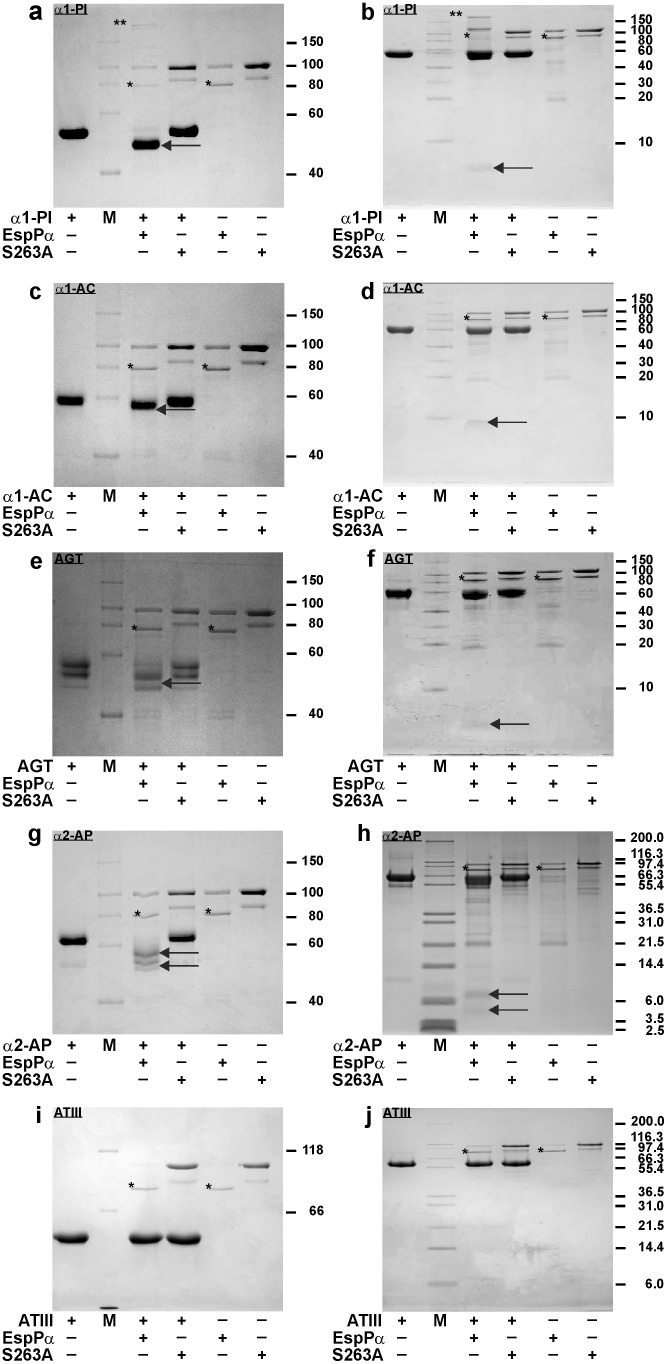
Figure 2. Cleavage of various serpins by EspPα.
Serpins (5 µg) were incubated (15 h, 37°C) with EspPα or S263A (1.5 µg). Degradation products were separated via SDS-PAGE using a glycine buffer (a, c, e, g, i) or tricine buffer system (b, d, f, h, j). Proteolytic serpin fragments formed by EspPα are indicated by an arrow. a, b α1-PI is degraded to a large and small fragment (∼45 kDa and ∼4 kDa, respectively), c, d cleavage of α1-AC in two fragments, e, f the AGT band with the highest molecular weight is cleaved in two fragments, g, h large and small fragments (∼55–57 kDa and ∼4–7 kDa) formed by α2-AP cleavage. i, j ATIII is not cleaved by EspPα. Incubation of α1-PI with EspPα leads to a weak formation of an inhibitory enzyme-serpin complex as marked by **. M, molecular weight marker, *, autodegradation product of EspPα.