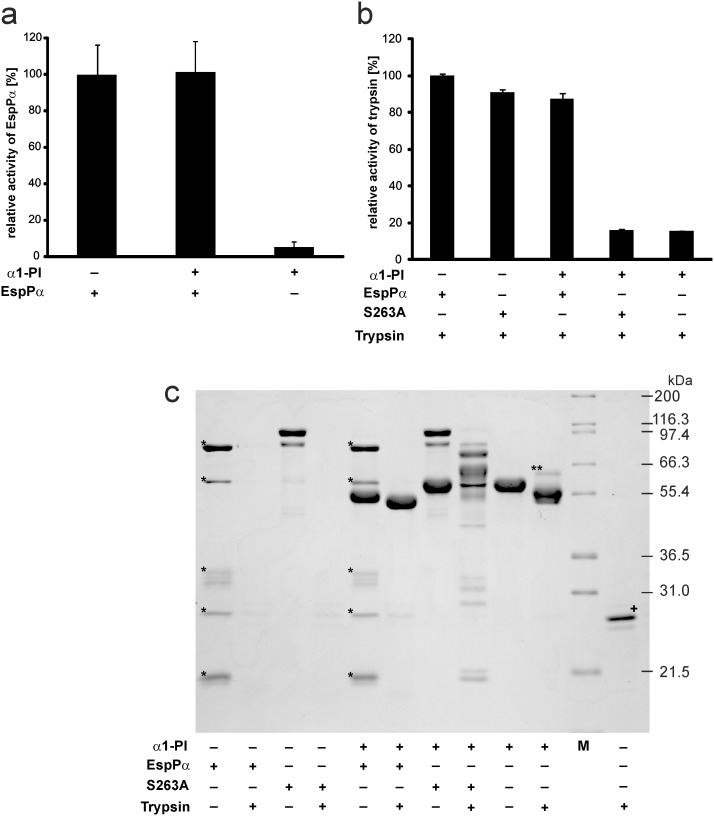
Figure 3. Activity of EspPα and α1-PI after coincubation.
a, Determination of EspPα activity. EspPα and α1-PI were preincubated (15 h, 37°C) at equimolar concentrations and remaining activity of EspPα was analyzed by incubation of an aliquot of the mixture with the chromogenic substrate Suc-Ala-Ala-Pro-Leu-pNA. Activity was measured via released para-nitroaniline and normalized to EspPα. n = 9 for EspPα and EspPα+α1-PI or n = 6 for α1-PI, respectively. b, α1-PI activity (measured as inhibitory potential on trypsin) after incubation with EspPα. α1-PI and EspPα or S263A were preincubated at a molar ratio of serpin∶enzyme = 4∶1. Remaining inhibitory activity of α1-PI on trypsin was analyzed by incubation at a molar ratio of α1-PI∶trypsin = 4∶1. Trypsin activity was measured via release of para-nitroaniline from the chromogenic substrate Bz-Arg-pNA. c, SDS-PAGE analysis of conincubations. α1-PI, EspPα, S263A, and trypsin were incubated as in b) and mixtures were separated via SDS-PAGE (12% SDS-PAGE gel, glycine buffer). M, molecular weight marker, *, EspPα autodegradation product, **, inhibitory complex of α1-PI and trypsin, +, trypsin was directly subjected to SDS-PAGE without incubation.