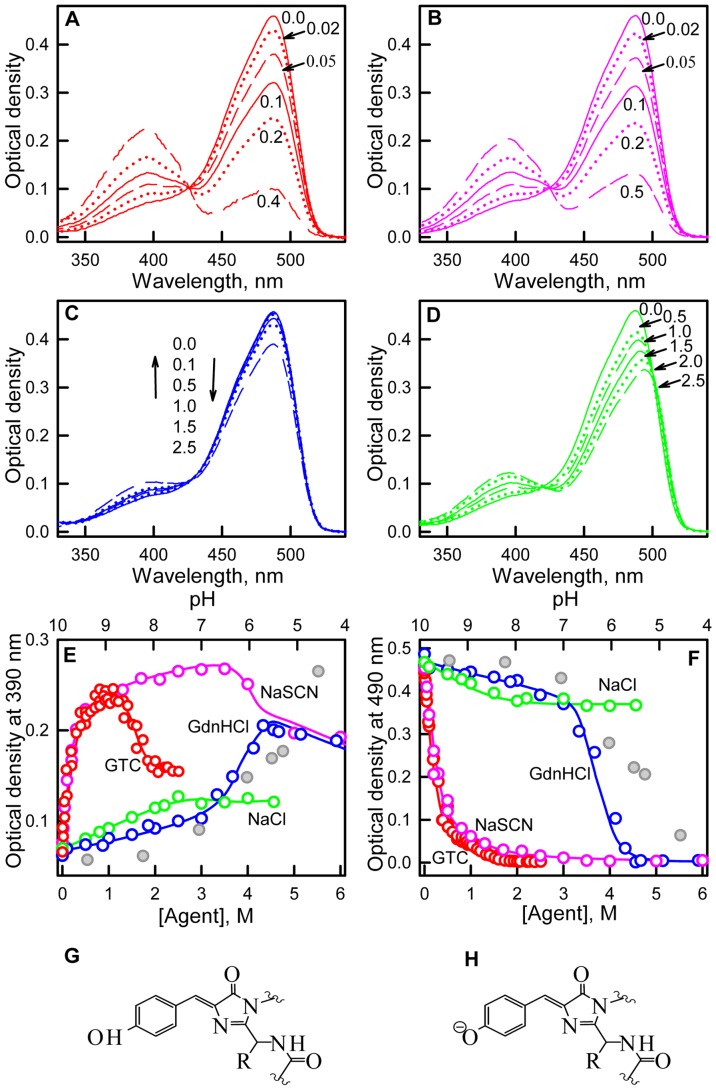
Figure 2. Ionic denaturants and salts effects on absorption of neutral and anionic chromophore forms of sfGFP.
(A) Absorption spectra of sfGFP in the buffered solution and in the presence of 0.02, 0.05, 0.1, 0.2 and 0.4 M GTC. (B) Absorption spectra of sfGFP in the buffered solution and in the presence of 0.02, 0.05, 0.1, 0.2 and 0.5 M NaSCN (C) Absorption spectra of sfGFP in the buffered solution and in the presence of 0.1, 0.5, 1.0, 1.5 and 2.5 M GdnHCl. (D) Absorption spectra of sfGFP in the buffered solution and in the presence of 0.5, 1.0, 1.5, 2.0 and 2.5 M NaCl (E) Changes in optical density at the absorption maxima for the neutral chromophore. (F) Changes in optical density at the absorption maxima for the anionic chromophore. Agents used included ionic denaturants, such as GTC (red circles) and GdnHCl (blue circles), and salts, such as NaSCN (pink circles) and NaCl (green circles). Data on absorption of neutral and anionic chromophore of sfGFP in different pH are shown in gray circles, axis of abscissas for these data is on top. Schematic representation of neutral (G) and anionic (H) chromophores is given.