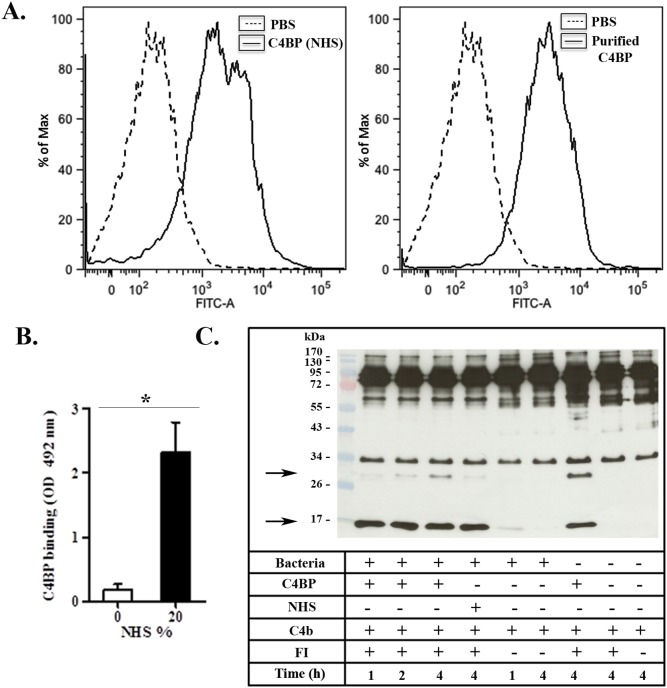
Figure 3. P. pneumotropica binds to C4b-binding protein and regulates the activation of the complement classical and lectin pathways.
(A) P. pneumotropica (108 bacteria/tube) was incubated with human C4-binding protein (C4BP) (1 µg) for 2 h or with normal human serum (NHS) for 30 min, followed by incubation with goat polyclonal antibody anti-C4BP (primary antibody) and then with anti-goat IgG-FITC conjugated (secondary antibody) (line histogram). As a negative control, untreated P. pneumotropica was incubated only with primary or secondary antibodies (dashed histogram). (B) Serum C4BP binding to P. pneumotropica was quantified by ELISA (*p<0.01). (C) Suspensions containing 2×108 bacteria/tube were incubated for 1 h at 37°C with C4BP (1 µg), or 10% NHS and then washed three times with PBS. C4b and FI (0.5 µg of each) were added to the bacterial suspensions pre-treated with C4BP, 10% NHS or PBS. These suspensions were then incubated at 37°C for 1, 2 or 4 h. The functional cofactor activity was evaluated by Western blot, and the cleavage fragments of C4b were detected using goat polyclonal primary anti-human C4. C4BP bound to P. pneumotropica acts as a co-factor of Factor I (FI) for the C4b cleavage. C4b cleavage fragments are indicated by arrows. Negative controls were included to observe spontaneous C4b cleavage in the absence of C4BP and/or FI.