Figure 7. Effects of βN265M mutation on etomidate in inactive versus active GABAA receptors.
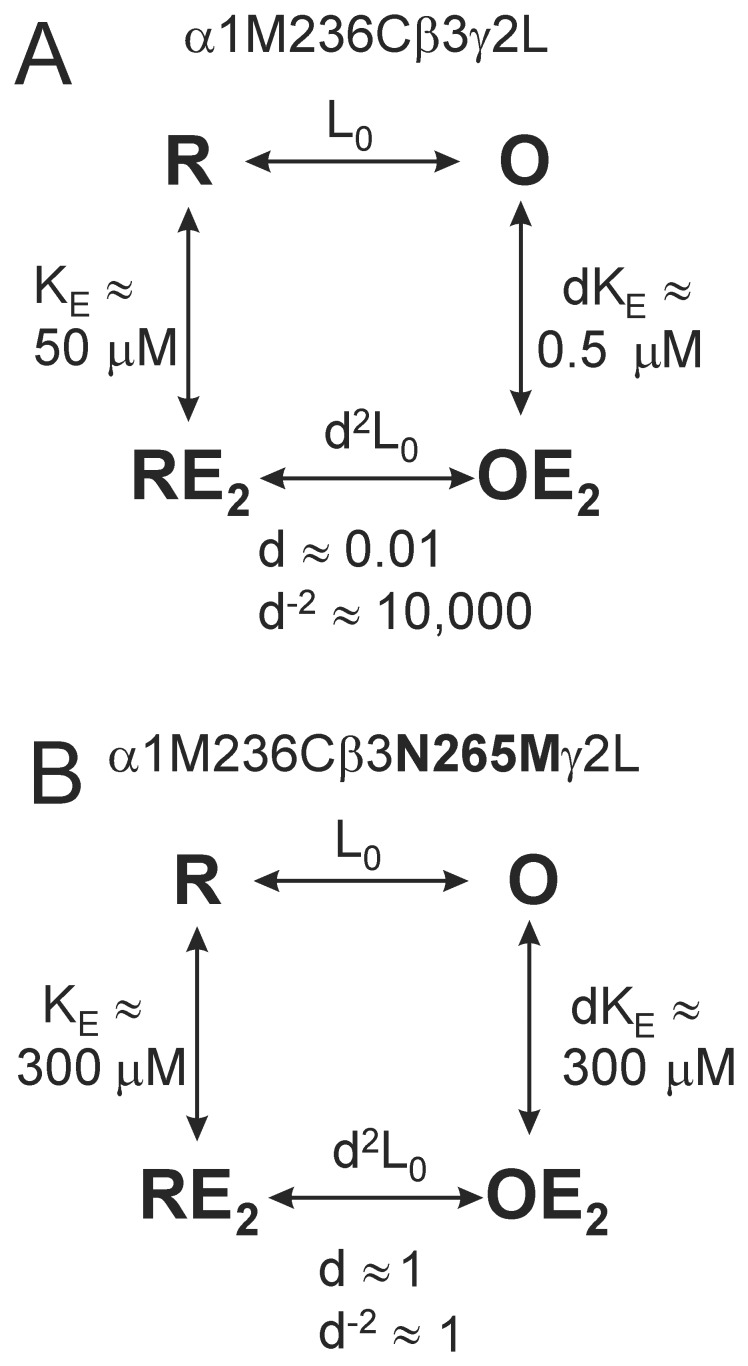
Monod-Wyman-Changeux equilibrium allosteric models are diagrammed for both α1M236Cβ2γ2L (A) and α1M236Cβ2N265Mγ2L (B) receptors, each with two equivalent etomidate sites. Etomidate dissociation constants for both inactive (R; KE) and GABA-activated (O; dKE) receptors are rounded estimates based on both functional analysis and modification/protection results (Figs. 6 and 7). Etomidate-sensitive α1M236Cβ2γ2L receptors bind etomidate 100-fold more avidly in the active vs. inactive state, resulting in a 10,000-fold shift in the open-closed equilibrium constant (d2L0 vs. L0) when both sites are occupied. In contrast, etomidate-insensitive α1M236Cβ2N265Mγ2L receptors display low affinity (KE ≈ dKE ≈ 300 µM) for etomidate in both resting and GABA-activated states. Thus, high etomidate concentrations result in only partial site occupancy and weak modulatory effects.