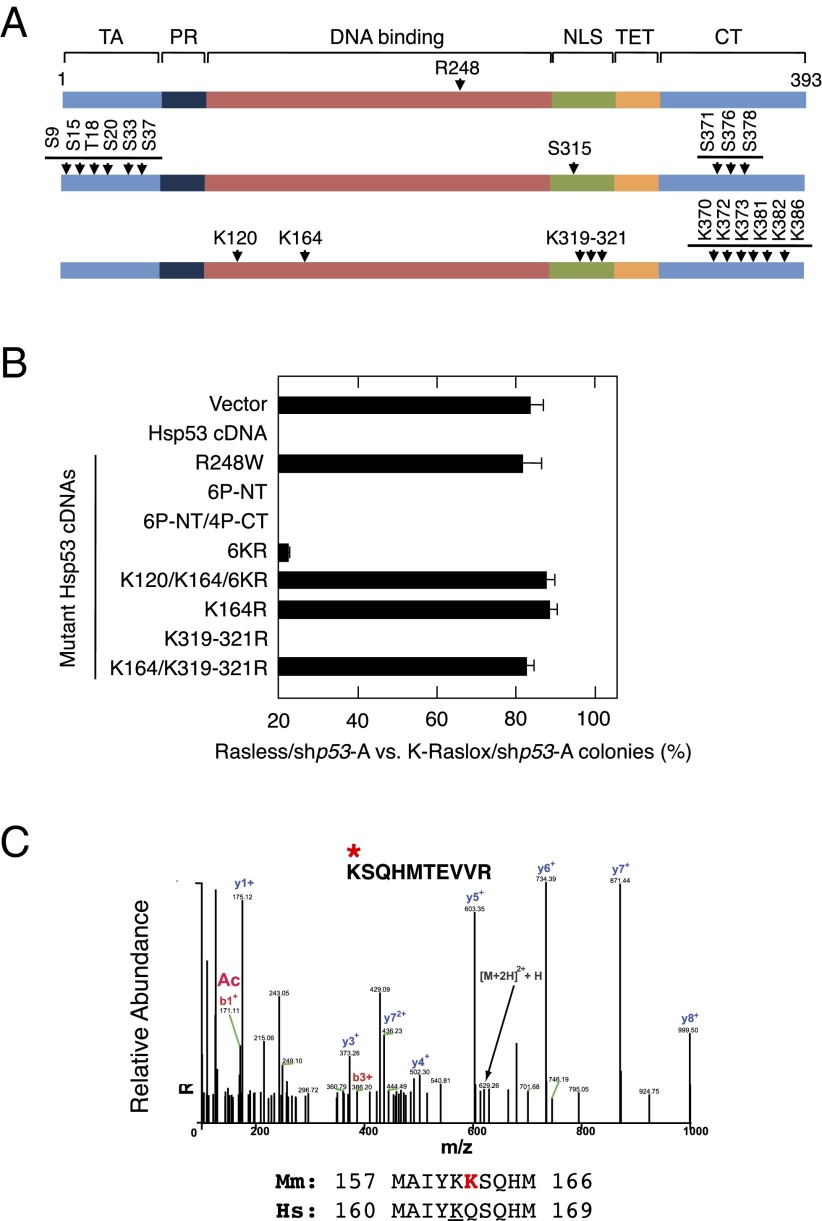
Fig. 4.
Requirement for p53 acetylation for cell cycle arrest in the absence of Ras signaling. (A) Schematic representation of the Hsp53 residues mutated in this study. Residues involved in DNA binding (Top), phosphorylation (Middle), and acetylation (Bottom) events are indicated. Functional domains of p53, including the transactivation domain (TA), the proline-rich region (PR), the DNA binding domain (DNA binding), the nuclear localization signal (NLS), the tetramerization domain (TET), and the C-terminal region (CT), are indicated. (B) Colony formation of Rasless and K-Raslox MEFs expressing the shp53-A shRNA and infected with an empty retrovirus (Vector) or retroviruses encoding the indicated cDNAs and expressed as the ratio between the number of colonies observed in cells lacking Ras proteins (Rasless) and those cells expressing K-Ras (K-Raslox). Retroviruses included those retroviruses encoding a WT Hsp53 cDNA and the following Hsp53 mutant cDNAs: R248W, 6P-NT (S9A/S15A/T18A/S20A/S33A/S37A), 6P-NT/4P-CT (S9A/S15AT18A/S20A/S33A/S37A/S315A/S371A/S376A/S378A), 6KR (K370R/K372R/K373R/K381R/K382R/K386R), K120/K164/6KR (K120R/K164R/K370R/K372R/K373R/K381R/K382R/K386R), K164R, K319-321R, and K164R/K319-321R. Data are represented as mean ± SD. (C) MS analysis of murine p53 (Mmp53) K162 acetylation in Rasless cells after adenoviral expression and pull-down of an Mmp53-GFP fusion protein. Alignment of mouse Mmp53 and Hsp53 amino acids surrounding K162 is shown.