Significance
Autism is characterized by diverse behavioral traits. Guided by theoretical considerations and empirical data, this paper develops the hypothesis that many of autism's salient traits may be manifestations of an underlying impairment in predictive abilities. This impairment renders an otherwise orderly world to be experienced as a capriciously “magical” one. The hypothesis elucidates the information-processing roots of autism and, thereby, can aid the identification of neural structures likely to be differentially affected. Behavioral and neural measures of prediction might serve as early assays of predictive abilities in infants, and serve as useful tools in intervention design and in monitoring their effectiveness. The hypothesis also points to avenues for further research to determine molecular and circuit-level causal underpinnings of predictive impairments.
Keywords: probabilistic processing, endophenotype, Markov models, theory, heterogeneity
Abstract
A rich collection of empirical findings accumulated over the past three decades attests to the diversity of traits that constitute the autism phenotypes. It is unclear whether subsets of these traits share any underlying causality. This lack of a cohesive conceptualization of the disorder has complicated the search for broadly effective therapies, diagnostic markers, and neural/genetic correlates. In this paper, we describe how theoretical considerations and a review of empirical data lead to the hypothesis that some salient aspects of the autism phenotype may be manifestations of an underlying impairment in predictive abilities. With compromised prediction skills, an individual with autism inhabits a seemingly “magical” world wherein events occur unexpectedly and without cause. Immersion in such a capricious environment can prove overwhelming and compromise one’s ability to effectively interact with it. If validated, this hypothesis has the potential of providing unifying insights into multiple aspects of autism, with attendant benefits for improving diagnosis and therapy.
1. The Hypothesis of Predictive Impairment in Autism
An essential component of a magical phenomenon is the lack of a discernible cause: An event that we are unable to predict happens “as if by magic.” Given how well-honed our predictive abilities are, magicians have to resort to clever contrivances to achieve their mystifying effects. However, if our predictive abilities were somehow to be compromised, then even mundane occurrences in the environment might appear magical. Although a brief magical performance is enjoyable, unrelenting immersion in it can be overwhelming. A magical world suggests lack of control and impairs one’s ability to take preparatory actions. It can result in outcomes such as those that constitute the autism phenotypes. This idea is the crux of the hypothesis we develop here.
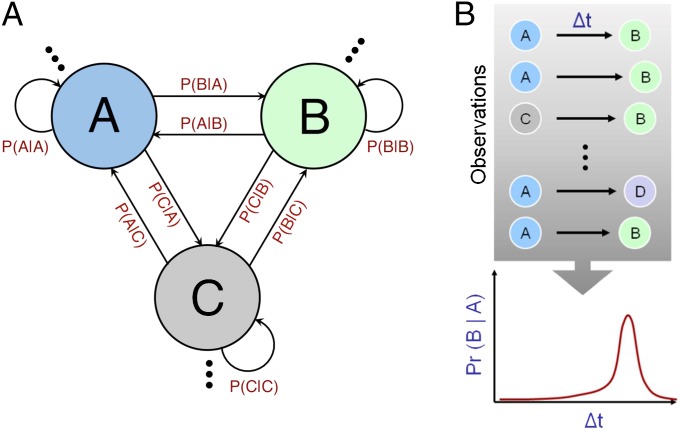
Theoretical motivation for the hypothesis that autism may be associated with a decreased ability to discern predictive relationships between environmental entities derives from the information processing demands inherent in the domains that are typically affected in autism. The primary diagnostic criteria for autism (1, 2)—social communication difficulties and restricted and repetitive behaviors—are superficially quite distinct from each other. However, they are quite similar from an information theoretic perspective: The underlying domains all constitute temporally unfolding Markov systems, with progressions from one state to another governed by probabilistic links (Fig. 1A). A large body of past work has shown how each of these domains maps onto such a formalism (3–13), albeit at different time-scales.
Fig. 1.
(A) A simple Markov system comprising probabilistically linked states. The domains that serve as diagnostic criteria for autism (language processing, social interactions, and behavioral repertoire) can all be modeled as temporally evolving Markov systems. The computation of transition probabilities is a key requirement for estimating a Markov system. (B) The task of transition probability estimation: from an observed temporal history of multiple state-to-state transitions, estimate P(B|A, Δt); the conditional probability of one state (“B”) given the other (“A”) and temporal duration, Δt, beyond A’s occurrence. The PIA hypothesis states that autism may be associated with inaccuracies in estimating this conditional probability and, hence, in one’s ability to discern predictive relationships between entities.
To perform well in these domains, one necessarily needs to estimate the underlying Markov system, i.e., the matrix of interstate transition probabilities. The fundamental prerequisite for such estimation is the extraction of state-to-state probabilities from observed event streams. As depicted in Fig. 1B, from an observed temporal sequence, the brain has to estimate the conditional probability P(B|A, Δt), the likelihood of transitioning to state “B” given the occurrence of “A” and elapsed temporal duration, Δt. The hypothesis of predictive impairment in autism (PIA) posits that autism may be associated with inaccuracies in estimating the P(B|A, Δt) conditional probability.
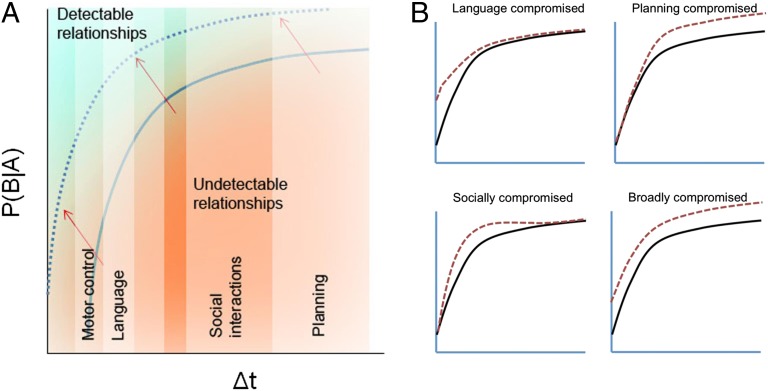
Fig. 2A depicts the PIA hypothesis schematically. Two key parameters characterize any interevent relationship: strength [P(B|A)] and temporal separation (Δt). In this 2D space, relationships toward the lower right may be undetectable, given that they have weak strength (B does not consistently follow A) and require integration over a large time interval (B occurs long after A has transpired). By contrast, relationships toward the upper left would be easier to detect. The association sensitivity function (ASF) defines the interface between detectable and undetectable relationships. A reduction in one’s predictive ability would, in this space, manifest as a shift of the ASF toward the upper left. As a consequence of this shift, several relationships that are evident to a neurotypical individual would become undetectable to a person with autism.
Fig. 2.
(A) A schematic depiction of the PIA hypothesis. Relationships between two events can be characterized by their strength [P(B|A)] and temporal separation (Δt). In this space, the interface between undetectable and detectable relationships marks the ASF, denoted by the solid curve here. The PIA hypothesis posits that autism is accompanied by a shift of the ASF toward the upper left (red arrows) corresponding to a reduction in one’s sensitivity to relationships that are weak and/or have large temporal spans. This shift renders some interevent relationships, which are evident to neurotypical individuals, invisible to those with autism. The vertical bands indicate that different tasks rely on the detection of interevent relationships over varying time-scales. For instance, whereas motor-control and language learning operate in the millisecond regime, social interactions and planning involve longer time intervals. (B) Behavioral manifestations of PIA may differ depending on which temporal regimes experience the greatest ASF shifts. As depicted in the four small graphs, different autism subtypes may arise in part from ASF shifts of different kinds.
Autism likely owes its genesis to a multiplicity of causes. We present the PIA hypothesis not as the sole causal factor underlying the condition, but rather as one that may provide an explanatory account of a few different aspects of the autism phenotype. Our hope is that as a theory, PIA may be able to help define some concrete avenues for further research into the causes of, and interventions for, autism. In the following sections, we consider the potential of this hypothesis to account for several important aspects of the autism phenotype (Section 2), its predictions regarding other traits one might expect to find in ASD (Section 3), and open questions and implications (Section 4).
2. The PIA Hypothesis as a Partial Account of the Autism Phenotype
Besides the aforementioned diagnostic domains, the hypothesis of impaired prediction can potentially account for a few other significant correlates of autism.
1. Insistence on Sameness.
Insistence on sameness (IoS) is a hallmark feature of autism. It is estimated that more than one-third of all individuals on the autism spectrum display some form of IoS (14). This trait may include repetitive thoughts and actions, behavioral rigidity, a reliance on routines, resistance to change, and obsessive adherence to rituals. Underscoring the significance of IoS as an attribute of the autism phenotype, the DSM-5 (15) incorporates IoS into its diagnostic criteria for the condition.
We can draw a compelling link between predictive impairments and insistence on rituals. Past research with diverse populations has shown that environmental unpredictability is strongly correlated with anxiety (16–20). Predictability is a fundamental modulator of anxiety in that reduction in the ability to predict events, even without any associated aversive consequence, enhances anxious responses (21, 22). Anxiety, especially when it is elevated chronically, is known to give rise to ritualistic behavior. These behaviors may be as benign as leg-swinging in school children who are working on a stressful math examination (23) or alarming stereotypies that may cause self-injury (24). Studies with neurologically healthy humans and animals reveal that the ritualistic behaviors that emerge under conditions of unpredictability serve as a calming response to an externally imposed stressor (25, 26). Taken together, these results suggest that rituals and an insistence on sameness may be a consequence of, and a way to mitigate, anxiety arising out of unpredictability.
First-person accounts by individuals on the autism spectrum are consistent with this possibility. For instance, Deborah Lipsky, a board member of the Autism Society of Maine says (27):
“I can’t emphasize enough how critical it is to understand that staying on a script is the sole means of keeping anxiety at a minimum. Even the smallest breach becomes a crisis because all we register at that moment is unpredictability. We fear unpredictability above all else because we are out of control of our environment.”
Completing the linkage to ritualistic behavior, Dora Raymaker, director of the Academic Autistic Spectrum Partnership in Research and Education, writes:
“The experience of many of us is not that ‘insistence on sameness’ jumps out unbidden and unwanted and makes our lives hard, but that “insistence on sameness” is actually a way of adapting to a confusing and chaotic environment…”
The same argument applies to stimming, a term used to refer to self-stimulating behaviors. The seemingly compulsive need to stim and their oftentimes social inappropriateness raises the possibility that these behaviors might be elaborate involuntary motor ticks. However, parental reports indicate that stimming behaviors are most evident in situations of heightened external stimulation (27), suggesting that stimming may be an anxiolytic response to a chaotic world: an attempt to drown out the influx of unpredictable environmental information by self-generated periodic and, hence, more predictable information. Consider this first-person account from Temple Grandin:
“When I did stims such as dribbling sand through my fingers, it calmed me down. When I stimmed, sounds that hurt my ears stopped. Most kids with autism do these repetitive behaviors because it feels good in some way. It may counteract an overwhelming sensory environment…”
Temple Grandin, Autism Asperger’s Digest, 2011
To summarize, a parsimonious interpretation of the reliance on rituals and stimming behaviors observed in autism is that they emerge from, and represent attempts to minimize, the consequences of unpredictability. They allow for a proactive imposition of “sameness” on an otherwise overwhelming environment.
2. Sensory Hypersensitivities.
It is estimated that nearly 90% of all children on the autism spectrum suffer from sensory abnormalities, often hypersensitivities, to stimuli that a neurotypical individual could easily ignore (28). These hypersensitivities cannot be explained as outcomes of abnormally enhanced sensation (29–31).
An alternative account of hypersensitivities comes from considering the complementary question: How do neurotypical individuals avoid being overwhelmed by sensory stimulation? A key role in suppressing sustained stimulation is played by our ability to habituate. A direct corollary is that reduced habituation reduces stimulus suppression. Immersion in an unrelentingly salient stimulus is known to be anxiogenic, as studies of the consequences of sensory bombardment have shown (32). Thus, aversion to environmental sounds that individuals with autism exhibit could arise from reduced habituation (33). However, this account begs the question, what kinds of factors might reduce habituation in autism?
A key determinant of habituation is stimulus predictability. For typically developing subjects, predictability of a sequence is directly proportional to the extent of habituation it induces (22, 34–36). Reduction in discerning predictive relationships between events in a stimulus sequence would reduce a person’s ability to predict the onset of the next event. Such a lack of predictability would compromise habituation and lead to hypersensitivity. Support for this idea can be found in a distasteful domain—torture techniques. Several studies have demonstrated that unpredictability of stressors is one of the key aspects of torture and leads to the development of anxiety, fear, and aversion (19, 37). “Acoustic bombardment” has long been used as an instrument of torture. Unfamiliar, and hence unpredictable, music is found to be especially effective (38). Tying this back to the domain of autism, the PIA hypothesis suggests that an endogenous predictive impairment causes environmental stimuli to appear more chaotic, leading to reduced habituation and hence greater stress.
3. Difficulties in Interacting with Dynamic Objects.
The dynamic world presents challenges to those with autism. The Autism Wandering and Elopement Initiative collaboration states that two in three “elopers” (autistic children who attempt to run away) have a close call with a traffic injury (39). According to the Center for Advanced Infrastructure and Transportation at Rutgers University, the vast majority (more than 75%) of people on the autism spectrum cannot drive (40). In first-person accounts, individuals with autism describe the difficulties they had as children engaging in dynamic games on the playground (41). These reports are puzzling given that moving objects are not inherently aversive to those with autism. Indeed, many children on the spectrum are especially drawn to moving objects, enjoy video games (42), obsessively set objects in motion, and even engage in visual stimming that generates movement. What then underlies autistic individuals’ difficulties with dynamic objects?
It is instructive to examine the specific nature of these difficulties. Several studies have shown that basic motion detection and direction perception thresholds are largely unimpaired in autism (43–45). However, to interact with a dynamic object, there is a crucial step beyond detection: anticipating where the moving object is likely to be so as to plan one’s motor movements appropriately to intercept/avoid the object. Even the seemingly simple task of keeping track of a moving object requires such anticipation; it is a manifestation of online Markov model estimation, as a visual temporal sequence unfolds. Computational systems for dynamic object tracking rely on predictive techniques such as Kalman filtering (46). These systems demonstrate that a key consequence of impaired prediction is errors in online position estimation. Transposed to the real world, this impairment would render seemingly straightforward tasks like avoiding a car or even catching a ball difficult for a person with autism.
Consider the following first person account:
Throwing a ball was not a problem. However, I couldn’t catch a ball until I was Age 13! I was criticized by my father about my ability, or lack of, to work with my hands.
(Jon Evans on www.DisabilityScoop.com)
Given the prediction-reliant nature of tasks requiring interactions with dynamic objects, the PIA hypothesis provides a plausible account of the difficulties that individuals with autism encounter in such settings.
4. Difficulties with Theory of Mind.
Theory of mind requires the ability to ascribe invisible causes to observations about a person by connecting past history with current behavior. Deducing why a person acted a certain way, or anticipating how a person is likely to act, requires the estimation of a conditional probability: Given a certain observation about a person and the circumstances, what are the likely precursors leading up to them, and in a similar vein, what are the next stages in their evolution? In this sense, Theory of mind is inherently a prediction task—given an observation, one has to postdict or predict its antecedent or subsequent states, i.e., estimate P(past history | current behavior) or P(future actions | current observations). Given the often-weak relationships among history, present observations, and future behavior for humans in social settings, these states involve the estimation of challenging probabilities and, hence, are especially vulnerable to impairments in predictive ability. Impairments in Theory of mind are a prominent correlate of autism (47).
Impairment in prediction would render an observer unable to situate current observations about a person in the context of their antecedents or likely future states. The observer, in effect, will be constrained to interpret observations “in the moment.” Such interpretations would manifest themselves as being literal, in that they describe what is happening from moment to moment, but are not influenced by past history and do not presage future events. The minimalistic movies of Heider and Simmel provide an interesting case in point (48). Having access to a probabilistic conditional relationship between observed movement patterns and potential histories, neurotypical individuals are able to ascribe causes to the former. The fact that these causes happen to be social in nature is likely happenstance; the social account is simply a better predictor of the observations than other historical accounts. An autistic individual, not having access to such predictive relationships, is constrained to interpret behaviors without any motivating history, social or otherwise (49).
In this way, the PIA hypothesis provides a simple account of the difficulties autistic individuals experience with theory of mind and other abstractions, which require the use of learned interevent relationships to read more into an observation than the basic sensory signal offers.
5. Islands of Proficiency.
Individuals with autism have been reported to show preserved, or even enhanced, abilities relative to neurotypical controls in several domains (50). These islands of proficiency include mathematics (51), static form coherence (52), visual search (53, 54), block design tasks (55), calendar calculations (56, 57), musical performance (50, 58), and drawing abilities (59, 60). These seemingly distinct domains share an important characteristic. Most of them, especially mathematics calendar calculations and music, are strongly rule-based, and, thus, minimize uncertainty of outcomes. Furthermore, with the exception of music, they are static, thereby obviating the need for prediction over time. If the prediction systems of the brain are differentially affected in autism, then one can expect that the skills left largely untouched would be the ones that are deterministic and where good performance does not depend on estimates of temporally unfolding conditional probabilities.
An examination of studies of preserved or enhanced proficiency in music (61, 62) reveals that the observed high performance is typically in replication. The reports further note that such factors as perfect pitch, prodigious memory, and extensive rehearsal, while contributing to the extraordinary skill set, are not sufficient to explain it (50, 58, 60). Detailed studies of musical savants with autism reveal that the basis for exceptional recall and performance, including improvisation, is familiarity with the structure of music and, thus, depends on adherence to rules (63).
As the foregoing discussion suggests, the PIA hypothesis provides a parsimonious account of some notable aspects of the autism phenotype. We next consider some empirically testable predictions this hypothesis makes about other traits that “should” be observed in individuals with autism.
3. Predictions Arising from the PIA Hypothesis
1. Hyperplasticity.
An important outcome of reduction in predictive abilities is a heightening of the perceived novelty of environmental stimuli. This enhanced novelty has a potentially interesting consequence on learning. It is known that activity in the brainstem and basal ganglia is modulated by novelty (64). Furthermore, significant evidence suggests that these structures play an important role in modulating learning (65, 66). Given the elevation of novelty due to impaired predictive skills, one would expect hyperactivation of the brainstem and basal ganglia in individuals with autism. Hyperarousal of these structures would, in turn, be expected to lead to hypermalleability of learning; current exposure would have disproportionate weight and supersede earlier experience, constituting a kind of “hyperplasticity.” Notably, such hyperplasticity would, in turn, further impair instance aggregation and, thus, an accurate estimation of probability values. Interestingly, precisely such hyperplasticity has been reported in mouse models of autism (67).
2. Changes in Striatum, Basal Ganglia, and Cerebellum.
In neurotypical individuals, the key brain loci that have been implicated in prediction are basal ganglia (68, 69), striatum (70, 71), anterior cingulate (72), and cerebellum (73, 74). If predictive abilities are affected in autism, then we would expect these regions of the brain to be especially implicated. Indeed, the emerging picture from recent neuro-imaging, postmortem, and animal-model studies is that atypicalities in these regions are associated with autism (75–80).
3. Reduced Appreciation of Humor.
A key component of humor is violation of expectation (81, 82). This implicitly requires the listener to have a prediction of how a given sequence is likely to unfold. A benign deviation from that prediction often constitutes a humorous outcome (82). In the absence of a strong prediction, a violation is harder to define and detect. Hence, opportunities for perceiving humor in narratives or observations are diminished. With this logic, the PIA hypothesis predicts that the sense of humor of individuals on the autism spectrum would be diminished or at least altered relative to that of neurotypicals.
Although systematic research on how autism affects the sense of humor is still in its infancy, some evidence supports this prediction. In describing his patients, Hans Asperger had noted that “an essential characteristic of these children is their humorlessness. They do not understand jokes…” (83). More recent studies (84, 85) have also documented these observations. It is particularly interesting to note Emerich et al.’s (86) finding that adolescents with autism had special difficulty “handling surprise and coherence within humorous narratives.”
4. Reduced Motor Anticipation.
Starting from a very early age, much of human motor behavior is anticipatory in nature. Whether it is adopting appropriate foot placement and body-posture while walking on uneven ground, or mouth shaping during speech articulation, there is extensive evidence for motor anticipation (87–89). The PIA hypothesis predicts that motor anticipation would be reduced in individuals with autism. The evidence so far is generally consistent with this prediction (90, 91). Even in his initial case reports, Kanner (92) noted poor anticipation on the part of autistic children to being picked-up by their parents. More recently, in a retrospective analysis Brisson et al. (93) have reported anticipatory failure during feeding situations in infants between 4 and 6 mo old who were later diagnosed as having autism. Fournier et al. (94), among others (95, 96), have found poor postural control in children with autism. Given that anticipatory processes have been implicated even in the maintenance of muscle tone (97), the PIA hypothesis might also help account for some aspects of hypotonia observed in children with autism.
4. Conclusion
Autism can have many etiologies, and yet, there is some commonality across manifestations, hence a shared clinical diagnosis. Based on theoretical considerations and a review of past literature, we believe that predictive impairment may constitute an endophenotype shared across individuals. This hypothesis may be a way to conceptualize a disorder that otherwise seems too heterogeneous and polymorphic. However, the notion of core impairments, shared by most cases of autism, seems to be at odds with the heterogeneity of the phenotype. How can the same basic impairment manifest itself so differently across different individuals? There are two points to note in responding to this concern.
First, despite the heterogeneity across cases of autism, there is also preserved commonality (98–100). Experienced clinicians are able to detect these core traits to reach their diagnostic assessments quickly. Thus, it would be misleading to think that the observed heterogeneity entirely overwhelms similarities. The PIA hypothesis is an attempt to characterize the shared aspects of autism. It is an idea similar in spirit to the recent finding of shared gene networks across many autism subtypes (101).
Second, the PIA hypothesis does not require that the predictive impairment be identical across all individuals; under the umbrella PIA hypothesis, there may well be parametric variations, as depicted in Fig. 2B. For instance, two children, both of whom have a predictive impairment, may differ in terms of the strength of predictive relationships they are able to detect, and the temporal regimes in which the predictive impairments are most pronounced. Impairments in the millisecond range might be more disruptive to language learning than those in the multisecond range, which may differentially affect social interactions instead. Such parametric differences may help account for the phenotypic variability observed across individuals on the autism spectrum and also potentially provide the basis for a principled taxonomy of different autism subtypes.
Another question that we have to consider in evaluating PIA is the need for such a hypothesis; Are there alternative explanations for aspects of the autism phenotype that we have tried to account for using PIA?
Explanatory accounts have indeed been offered for each of the autistic traits we have described in Sections 2 and 3. For instance, difficulties in Theory of Mind have been suggested as arising from abnormalities of the cortical substrates for social cognition (102) or in the mirror neuron system (refs. 103 and 104 but see 105). Motor difficulties are believed to arise from abnormalities in the cerebellum (106). The list goes on. The general point is that explanations exist, but are typically tied to specific traits. Although it is certainly possible that the different autistic traits have different etiologies, what is appealing about the PIA hypothesis is its parsimony—an account that is able to provide a unifying explanation for not just a single trait, but for several of them. Indeed, some recent data show that superficially very different aspects of the autism phenotype may, in fact, be linked. Cathy Lord and her coworkers (107) have found that the ability to throw or catch a ball is correlated with social skills success in children with autism. Although not conclusive evidence, this further lends support to the possibility of shared causality for seemingly different behavioral traits.
The hypothesis of a predictive impairment may not only help us better understand the information processing roots of the autism disorder, but also serve as a precursor to identifying the brain regions that are involved in these probabilistic processes and, perhaps, are differentially affected in autism. This program of research can build on the exciting body of recent work that has been investigating the prediction and reward machinery in the neurotypical brain, both in humans and other animals (108). Moving further down the causal chain, the methods of behavioral genetics can help examine genetic underpinnings of predictive abilities and, thereby, suggest candidate risk genes for autism. These studies could prove to be useful complements to those already underway by using linkage and copy number variation analyses (e.g., ref. 109). This approach would enable linkages to be made between the manifest traits of autism and the underlying genetic architecture. Reliable behavioral tests and neural markers of prediction may serve as early assays of these abilities in infants at risk and also be useful as tools to monitor the effectiveness of therapeutic interventions (as demonstrated in ref. 110).
Perhaps one beneficial outcome of this work will be to balance the sense of being overwhelmed by a relentlessly magical world with the feeling of tedium in the frequently mundane world of neurotypicals. By occasionally getting a glimpse of both worlds, we may be able to enrich all lives.
Acknowledgments
We thank Drs. Helen Tager-Flusberg, Charles Nelson, and Geraldine Dawson for discussions and several parents of children with autism for comments. This work was supported by the Simons Center for the Social Brain at Massachusetts Institute of Technology and by the Simons Foundation for Autism Research.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lord C, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 2.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 3.Natale M. A Markovian model of adult gaze behavior. J Psycholinguist Res. 1976;5(1):53–63. doi: 10.1007/BF01067948. [DOI] [PubMed] [Google Scholar]
- 4.Manderscheid RW, Rae DS, McCarrick AK, Silbergeld S. A stochastic model of relational control in dyadic interaction. Am Sociol Rev. 1982;47:62–75. [Google Scholar]
- 5.Thomas AP. Conversational routines: A Markov chain analysis. Lang Commun. 1985;5:287–296. [Google Scholar]
- 6.Crown CL, et al. Mathematical models for coordinated interpersonal timing in mother-infant interactions in the first year of life. J Psycholinguist Res. 1996;25(6):617–628. doi: 10.1007/BF01712412. [DOI] [PubMed] [Google Scholar]
- 7.Pentland A, Liu A. Modeling and prediction of human behavior. Neural Comput. 1999;11(1):229–242. doi: 10.1162/089976699300016890. [DOI] [PubMed] [Google Scholar]
- 8.Olekalns M, Smith P, Weingart L. Markov chain models of communication processes in negotiation. Int Negot. 2005;10:97–114. [Google Scholar]
- 9.Wang H-C. 2008. Modeling idea generation sequences using hidden Markov models. Cognitive Science Conference Proceedings (Cognitive Science Society, Washington) pp 107–112.
- 10.Chung PC, De Liu C. A daily behavior enabled hidden Markov model for human behavior understanding. Pattern Recognit. 2008;41:1589–1597. [Google Scholar]
- 11.Behrens TEJ, Hunt LT, Rushworth MFS. The computation of social behavior. Science. 2009;324(5931):1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- 12.Heerey EA, Crossley HM. Predictive and reactive mechanisms in smile reciprocity. Psychol Sci. 2013;24:1446–1455. doi: 10.1177/0956797612472203. [DOI] [PubMed] [Google Scholar]
- 13.Fisher BA, Drecksel GL, Werbel WS. Social Information Processing Analysis (SIPA): Coding ongoing human communication. Small Group Behav. 1979;10:3–21. [Google Scholar]
- 14.Gray KM, Tonge BJ. Screening for autism in infants and preschool children with developmental delay. Aust N Z J Psychiatry. 2005;39(5):378–386. doi: 10.1080/j.1440-1614.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association 2013. Diagnostic and Statistical Manual of Mental Disorders, (American Psychiatric Publishing, Arlington, VA), 5th Ed.
- 16.Tsuda A, Ida Y, Satoh H, Tsujimaru S, Tanaka M. Stressor predictability and rat brain noradrenaline metabolism. Pharmacol Biochem Behav. 1989;32(2):569–572. doi: 10.1016/0091-3057(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 17.Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55(11):1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- 18.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: An animal model. Psychol Bull. 1992;112(2):218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 19.Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: A new perspective on experimental neurosis. J Abnorm Psychol. 1978;87(2):256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- 20.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33(2):320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 21.Abbott BB, Badia P. Predictable versus unpredictable shock conditions and physiological measures of stress: A reply to Arthur. Psychol Bull. 1986;100:384–387. [Google Scholar]
- 22.Herry C, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27(22):5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soussignan R, Koch P. Rhythmical stereotypies (leg-swinging) associated with reductions in heart-rate in normal school children. Biol Psychol. 1985;21(3):161–167. doi: 10.1016/0301-0511(85)90027-4. [DOI] [PubMed] [Google Scholar]
- 24.Fox MW. 1986. Laboratory Animal Husbandry: Ethology, Welfare, and Experimental Variables (State Univ of New York Press, Albany)
- 25.Maier NRF. Studies of Abnormal Behavior in the Rat. Harper; New York: 1939. [Google Scholar]
- 26.Eilam D, Izhar R, Mort J. Threat detection: Behavioral practices in animals and humans. Neurosci Biobehav Rev. 2011;35(4):999–1006. doi: 10.1016/j.neubiorev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Lipsky D. From Anxiety to Meltdown: How Individuals on the Autism Spectrum Deal with Anxiety, Experience Meltdowns, Manifest Tantrums, and How You Can Intervene Effectively. Jessica Kingsley Publishers; London: 2011. pp. 74–75. [Google Scholar]
- 28.Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37(5):894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- 29.DePape A-MR, Hall GBC, Tillmann B, Trainor LJ. Auditory processing in high-functioning adolescents with autism spectrum disorder. PLoS ONE. 2012;7(9):e44084. doi: 10.1371/journal.pone.0044084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bölte S, et al. A close eye on the eagle-eyed visual acuity hypothesis of autism. J Autism Dev Disord. 2012;42(5):726–733. doi: 10.1007/s10803-011-1300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashwin E, Ashwin C, Rhydderch D, Howells J, Baron-Cohen S. Eagle-eyed visual acuity: An experimental investigation of enhanced perception in autism. Biol Psychiatry. 2009;65(1):17–21. doi: 10.1016/j.biopsych.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Plutchik R. The effects of high intensity intermittent sound on performance, feeling and physiology. 1959;56:133–151. doi: 10.1037/h0047477. [DOI] [PubMed] [Google Scholar]
- 33.Kleinhans NM, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 34.Sokolov EN. The Central Nervous System and Behavior III. Macy Foundation; New York: 1960. [Google Scholar]
- 35.McDaniel JW. 1964. The effects of stimulus predictability upon auditory habituation and acquisition. Dissertation (Texas Technical College, Lubbock, TX)
- 36.Turk-Browne NB, Scholl BJ, Chun MM. Babies and brains: Habituation in infant cognition and functional neuroimaging. Front Hum Neurosci. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Başoğlu M, Mineka S. 1992. The role of uncontrollable and unpredictable stress in post-traumatic stress responses in torture survivors. Torture and Its Consequences: Current Treatment Approaches (Cambridge Univ Press, Cambridge, UK), pp 185–255.
- 38.Senior J. 2009. PsyOps Rock! New York Mag. Available at www.nymag.com. Accessed October 23, 2009.
- 39. AWAARE Collaboration (2014) Autism and Wandering. Available at: www.awaare.org.
- 40.Feeley C. 2010. Evaluating the transportation needs and accessibility for adults on the autism spectrum in New Jersey. Transp Res Board 8(Jan):1–24.
- 41.Robison JE. Look Me in the Eye: My Life with Asperger’s. Three Rivers; New York: 2006. [Google Scholar]
- 42.Mazurek MO, Shattuck PT, Wagner M, Cooper BP. Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. J Autism Dev Disord. 2012;42(8):1757–1767. doi: 10.1007/s10803-011-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: A “complex” issue. J Cogn Neurosci. 2003;15(2):218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- 44.Swettenham J, Campbell R. Motion perception and autistic spectrum disorder : A review. Curr Psychol Cogn. 2005;23:3–33. [Google Scholar]
- 45.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 46.Welch G, Bishop G. 2001. An introduction to the Kalman Filter. Proc SIGGRAPH 2001 (Association for Computing Machinery, Los Angeles), pp 19–24.
- 47.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 48.Heider F, Simmel M. An experimental study of apparent behavior. Am J Psychol. 1944;57:243–259. [Google Scholar]
- 49.Klin A. Attributing social meaning to ambiguous visual stimuli in higher-functioning autism and Asperger syndrome: The Social Attribution Task. J Child Psychol Psychiatry. 2000;41(7):831–846. [PubMed] [Google Scholar]
- 50.Wallace GL. Neuropsychological studies of savant skills: Can they inform the neuroscience of giftedness? Roeper Rev. 2008;30:229–246. [Google Scholar]
- 51.Iuculano T, et al. Brain organization underlying superior mathematical abilities in children with autism. Biol Psychiatry. 2014;75(3):223–230. doi: 10.1016/j.biopsych.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer J, et al. Motion processing in autism: Evidence for a dorsal stream deficiency. Neuroreport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- 53.O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. J Exp Psychol Hum Percept Perform. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez C, Martin JM, Minshew NJ, Behrmann M. Practice makes improvement: How adults with autism out-perform others in a naturalistic visual search task. J Autism Dev Disord. 2013;43(10):2259–2268. doi: 10.1007/s10803-013-1772-4. [DOI] [PubMed] [Google Scholar]
- 55.Shah A, Frith U. An islet of ability in autistic children: A research note. J Child Psychol Psychiatry. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 56.Kehrer HE. Savant capabilities of autistic persons. Acta Paedopsychiatr. 1992;55(3):151–155. [PubMed] [Google Scholar]
- 57.Kelly SJ, Macaruso P, Sokol SM. 1997. Mental calculation in an autistic savant: A case study. J Clin Exp Neuropsychol 19(2):172–184. [DOI] [PubMed]
- 58.Rimland B, Hill AL. In: Mental Retardation and Developmental Disabilities. Wortis J, editor. Pleneum; New York: 1984. pp. 155–169. [Google Scholar]
- 59.Hermelin B. Bright Splinters of the Mind. A Personal Story of Research with Autistic Savants. J. Kingsley; London: 2001. [Google Scholar]
- 60.Pring L. Savant talent. Dev Med Child Neurol. 2005;47(7):500–503. doi: 10.1017/s0012162205000976. [DOI] [PubMed] [Google Scholar]
- 61.Miller L. Musical Savants: Exceptional Skill in the Mentally Retarded. Psychology; New York: 1989. [Google Scholar]
- 62.Ōe K. A Healing Family. Kodansha International; Tokyo: 1996. [Google Scholar]
- 63.Young RL, Nettelbeck T. The abilities of a musical savant and his family. J Autism Dev Disord. 1995;25(3):231–248. doi: 10.1007/BF02179286. [DOI] [PubMed] [Google Scholar]
- 64.Joshua M, Adler A, Bergman H. Novelty encoding by the output neurons of the Basal Ganglia. Front Syst Neurosci. 2010;3:20. doi: 10.3389/neuro.06.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 66.Sutton RS, Barto AG. Reinforcement Learning: An Introduction. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 67.Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic acid animal model of autism. Front Neural Circuits. 2008;2:4. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz W, Tremblay L, Hollerman JR. 1998. Reward prediction in primate basal ganglia and front cortex. Neuropharmacology 37(4–5):421–429.
- 69.Tanaka SC, et al. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7(8):887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 70.Cooper JC, Dunne S, Furey T, O’Doherty JP. Human dorsal striatum encodes prediction errors during observational learning of instrumental actions. J Cogn Neurosci. 2012;24(1):106–118. doi: 10.1162/jocn_a_00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc Lond B Biol Sci. 2008;363(1511):3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ide JS, Shenoy P, Yu AJ, Li CS. Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci. 2013;33(5):2039–2047. doi: 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bastian AJ. Learning to predict the future: The cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16(6):645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Schlerf J, Ivry RB, Diedrichsen J. Encoding of sensory prediction errors in the human cerebellum. J Neurosci. 2012;32(14):4913–4922. doi: 10.1523/JNEUROSCI.4504-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thakkar KN, et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131(Pt 9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peça J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH. Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):539–551, e1–e4. doi: 10.1016/j.jaac.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 78.Sears LL, et al. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 79.Fatemi SH, et al. Consensus paper: Pathological role of the cerebellum in autism. Cerebellum. 2012;11(3):777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai PT, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488(7413):647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veatch TC. A theory of humor. Humor. 1998;11:161–215. [Google Scholar]
- 82.McGraw AP, Warren C. Benign violations: Making immoral behavior funny. APS. 2010;21:1141–1149. doi: 10.1177/0956797610376073. [DOI] [PubMed] [Google Scholar]
- 83.Asperger H. Die “Autistischen Psychopathen” im Kindesalter. Eur Arch Psychiatry Clin Neurosci. 1944;117:76–136. [Google Scholar]
- 84.Lyons V, Fitzgerald M. Humor in autism and Asperger syndrome. J Autism Dev Disord. 2004;34(5):521–531. doi: 10.1007/s10803-004-2547-8. [DOI] [PubMed] [Google Scholar]
- 85.Samson AC, Huber O, Ruch W. Seven decades after Hans Asperger’s observations: A comprehensive study of humor in individuals with Autism Spectrum Disorders. Humor. 2013;26:441–460. [Google Scholar]
- 86.Emerich DM, Creaghead NA, Grether SM, Murray D, Grasha C. The comprehension of humorous materials by adolescents with high-functioning autism and Asperger’s syndrome. J Autism Dev Disord. 2003;33(3):253–257. doi: 10.1023/a:1024498232284. [DOI] [PubMed] [Google Scholar]
- 87.Hugon M, Massion J, Wiesendanger M. Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflugers Arch. 1982;393(4):292–296. doi: 10.1007/BF00581412. [DOI] [PubMed] [Google Scholar]
- 88.Reddy V, Markova G, Wallot S. Anticipatory adjustments to being picked up in infancy. PLoS ONE. 2013;8(6):e65289. doi: 10.1371/journal.pone.0065289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ambrosini E, et al. Looking ahead: Anticipatory gaze and motor ability in infancy. PLoS ONE. 2013;8(7):e67916. doi: 10.1371/journal.pone.0067916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes C. Brief report: Planning problems in autism at the level of motor control. J Autism Dev Disord. 1996;26(1):99–107. doi: 10.1007/BF02276237. [DOI] [PubMed] [Google Scholar]
- 91.Schmitz C, Martineau J, Barthélémy C, Assaiante C. Motor control and children with autism: Deficit of anticipatory function? Neurosci Lett. 2003;348(1):17–20. doi: 10.1016/s0304-3940(03)00644-x. [DOI] [PubMed] [Google Scholar]
- 92.Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35(4):100–136. [PubMed] [Google Scholar]
- 93.Brisson J, Warreyn P, Serres J, Foussier S, Adrien-Louis J. Motor anticipation failure in infants with autism: A retrospective analysis of feeding situations. Autism. 2012;16(4):420–429. doi: 10.1177/1362361311423385. [DOI] [PubMed] [Google Scholar]
- 94.Fournier KA, et al. Decreased static and dynamic postural control in children with autism spectrum disorders. Gait Posture. 2010;32(1):6–9. doi: 10.1016/j.gaitpost.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with autism spectrum disorder. J Autism Dev Disord. 2003;33(6):643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- 96.Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- 97.Vishton PM, Reardon KM, Stevens JA. Timing of anticipatory muscle tensing control: Responses before and after expected impact. Exp Brain Res. 2010;202(3):661–667. doi: 10.1007/s00221-010-2172-z. [DOI] [PubMed] [Google Scholar]
- 98.Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones W, Klin A. Heterogeneity and homogeneity across the autism spectrum: The role of development. J Am Acad Child Adolesc Psychiatry. 2009;48(5):471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- 101.Voineagu I, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takeuchi M, Harada M, Nishitani H. Deficiency of “theory of mind” in autism estimated by fMRI. Int Congr Ser. 2002;1232:737–740. [Google Scholar]
- 103.Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- 104.Hadjikhani N. 2007. Mirror neuron system and autism Progress in Autism Research, ed Carlisle PC, (Nova Science Publishers, Happauge, NY), pp 151–166.
- 105.Dinstein I, et al. Normal movement selectivity in autism. Neuron. 2010;66(3):461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mostofsky SH, et al. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(Pt 9):2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MacDonald M, Lord C, Ulrich DA. The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapt Phys Activ Q. 2013;30(3):271–282. doi: 10.1123/apaq.30.3.271. [DOI] [PubMed] [Google Scholar]
- 108.Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 109.Szatmari P, et al. Autism Genome Project Consortium Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dawson G, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]