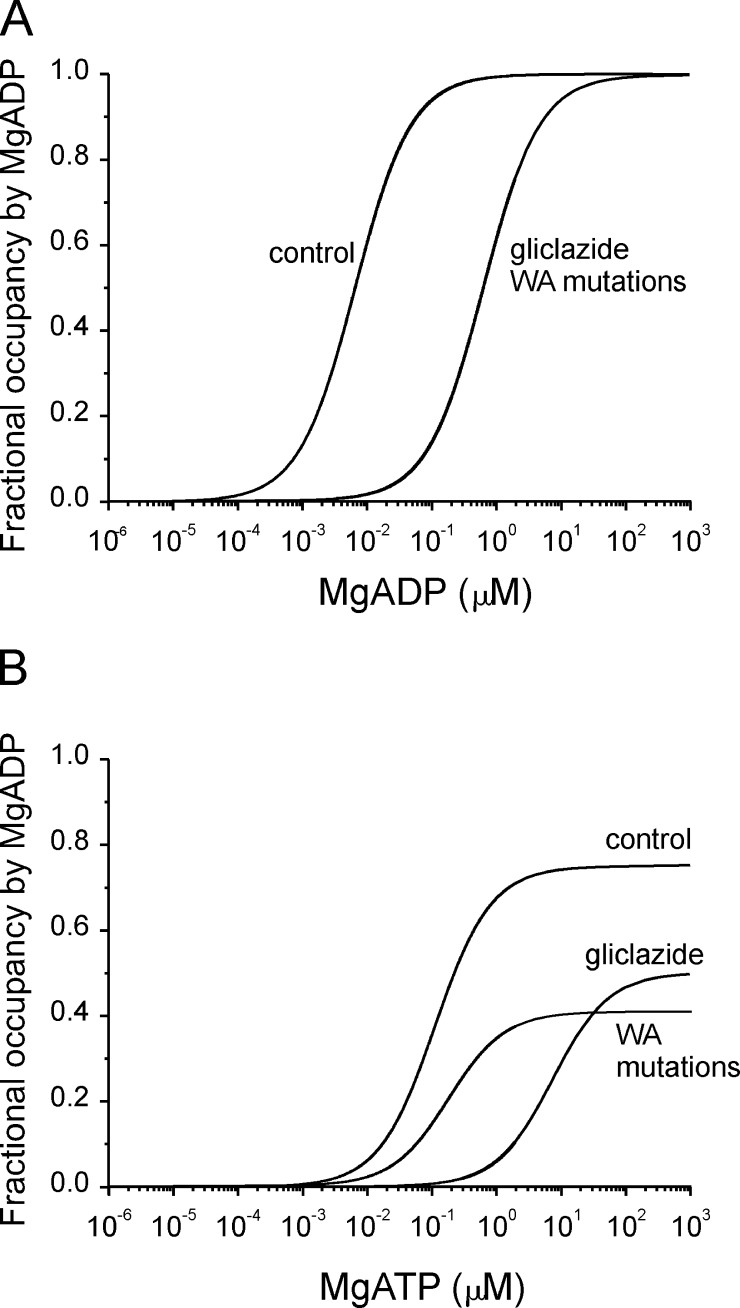
Figure 9.
Simulations of the fractional occupancy of NBS2 by MgADP. (A and B) Simulations of the fractional occupancy of NBS2 by MgADP in the presence of either MgADP (A; Eq. 5) or MgATP (B; Eq. 6). The model and the values of the rate constants were taken from Bienengraeber et al. (2004). Calculated control curves for MgADP and MgATP predicted EC50 values for MgADP occupancy of NBS2 that were very similar to those measured experimentally for channel activation. (A) To simulate the effect of gliclazide or the K1A and K2A mutations (KA mutations) on MgADP activation, we assumed a threefold increase in the off rate for MgADP binding in the presence of gliclazide (as measured experimentally) or when K1A or K2A was mutated. A 30-fold decrease in the on rate of MgADP binding in the presence of gliclazide (or the K1A or K2A mutation) predicted the measured EC50 for MgADP activation in the presence of gliclazide. (B) We used the same values for the rate constants for MgADP binding as in A and assumed the binding affinity of MgATP was reduced by gliclazide to the same extent as MgADP binding. This resulted in a predicted EC50 for the fractional occupancy of NBS2 by MgADP in the presence of gliclazide of 8 mM, which was the same as that measured experimentally for MgATP activation. To model the effect of the K1A and K2A mutations, we assumed the mutations had no effect on MgATP binding (as observed experimentally) and that they reduced the rate of ATP hydrolysis 100-fold. This reduced the maximal fractional occupancy of NBS2 by MgADP, but had no effect on the EC50 (as observed experimentally).