Figure 7.
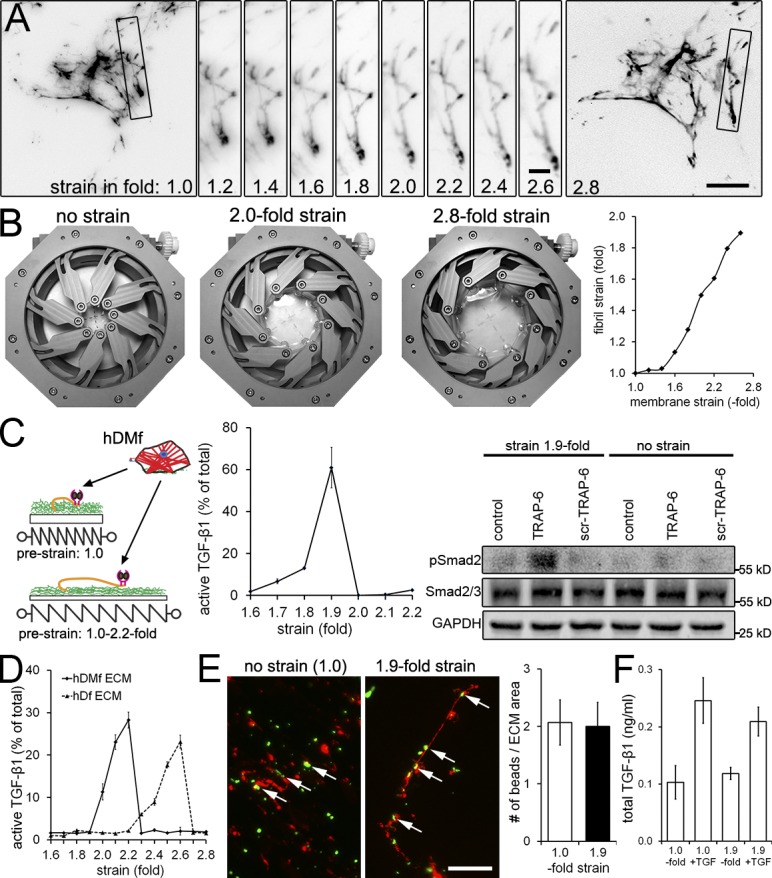
Prestraining LTBP-1–containing ECM enhances subsequent TGF-β1 release. hDMfs grown on relaxed highly expandable silicone membranes were removed after 6 d using DOC. (A) The cell-free DOC-insoluble ECM was stained without fixation for LTBP-1 and visualized during membrane expansion from relaxed (onefold) to 2.8-fold strains. Selected fibrils (boxes) were magnified and followed in incremental steps of a 0.2-fold membrane strain. (B) Illustration of the strain device opening and membrane expansion. Strain of LTBP-1–containing fibrils was measured as fold length change compared with initial length and plotted against membrane surface expansion. The data shown are from a single representative experiment out of three repeats. (C) hDMfs were seeded onto nonstrained (onefold) and prestrained decellularized ECM (≤2.2-fold). Cell contraction was induced in every condition using thrombin and TRAP-6 in select conditions, and release of active TGF-β1 was quantified as the percentage of total TGF-β1. Western blotting was performed for phospho- and total Smad2 in subsequently lysed myofibroblasts. (D) The decellularized ECM produced by either hDMfs or hDfs was strained in the absence of cells by expanding the membrane ≤2.8-fold, and active TGF-β1 released into the supernatant was measured at every 0.1-fold increment. (E) Decellularized ECM labeled for LTBP-1 (red) and green fluorescent microspheres at strains of 1.0- and 2.8-fold. Arrows indicate microspheres bound to LTBP-1 fibrils. The number of microspheres in the image field was quantified and normalized to the area covered by LTBP-1 fibrils. (F) 1 ng/ml active TGF-β1 was added for 1 h to cell-free hDMf-derived ECM that was either nonstrained (onefold) or strained 1.9-fold. Samples were rigorously washed three times, ECM was subsequently lysed, and levels of ECM-contained TGF-β1 were measured using TMLCs. The graph shows mean values and SDs from at least three independent experiments. Bars: (main images) 20 µm; (magnified images) 4 µm.
