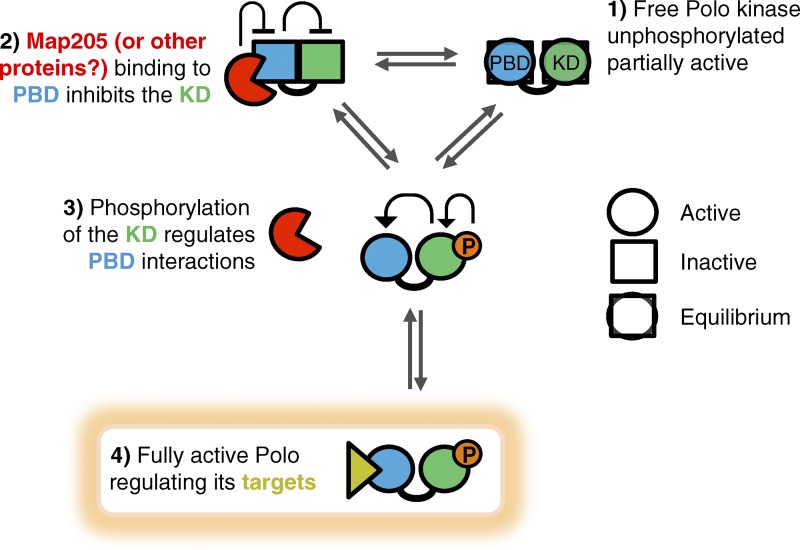
Figure 5.
Model for the interdomain allosteric regulation of Polo. (1) Free, unphosphorylated Polo is partially active. (2) Binding of the inhibitor Map205 to the PBD stabilizes its closed conformation, which stabilizes the inactive conformation of the KD. (3) Conversely, T-loop phosphorylation of the KD induces a conformation change that destabilizes its interaction with the PBD, thereby destabilizing its Map205-binding conformation. (4) The PBD can then adopt its conformation, which is competent for binding canonical targets.