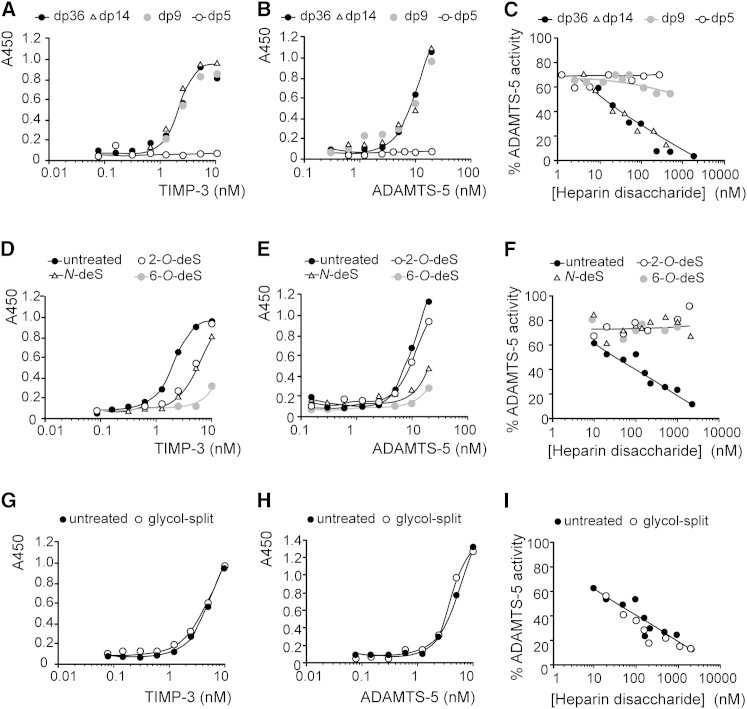
Figure 5.
Size and Sulfation Affect the Heparin-Mediated Increase in TIMP-3 Affinity for ADAMTS-5
(A and B) Heparin (60 μM disaccharide) of dp36 (12.5 kDa), dp14 (5 kDa), dp9 (3 kDa), or dp5 (1.7 kDa) was immobilized on glycosaminoglycan-binding multiwell plates. The wells were then incubated with FLAG-tagged TIMP-3 (0.08–10 nM) (A) or FLAG-tagged ADAMTS-5 (0.3–20 nM) (B). Bound protein was detected using M2 anti-FLAG primary antibody and a horseradish peroxidase-coupled secondary antibody.
(C) TIMP-3 (0.5 nM) and ADAMTS-5 (0.5 nM) were incubated (1 hr, 37°C) with various concentrations (1–2000 nM disaccharide) of heparin with dp36, dp14, dp9, or dp5. The residual activity against the fluorescent peptide substrate was determined.
(D and E) Heparin (60 μM disaccharide) of dp36 (12.5 kDa) either normally sulfated (untreated), N-desulfated (N-deS), 6-O-desulfated (6-O-deS), or 2-O-desulfated (2-O-deS) was immobilized on glycosaminoglycan-binding multiwell plates. The wells were then incubated with FLAG-tagged TIMP-3 (0.08–10 nM) (D) or FLAG-tagged ADAMTS-5 (0.16–20 nM) (E). Bound protein was detected using M2 anti-FLAG primary antibody and a horseradish peroxidase-coupled secondary antibody.
(F) TIMP-3 (0.5 nM) and ADAMTS-5 (0.5 nM) were incubated (1 hr, 37°C) with various concentrations (10–2000 nM disaccharide) of dp36 (12.5 kDa) heparin, either normally sulfated (untreated), N-desulfated, 6-O-desulfated, or 2-O-desulfated The residual activity against the fluorescent peptide substrate was determined.
(G and H) Heparin of dp36 (untreated) or glycol-split dp36 heparin (60 μM disaccharide) were immobilized on glycosaminoglycan-binding multiwell plates, and the wells were then incubated with FLAG-tagged TIMP-3 (0.08–10 nM) (G) or FLAG-tagged ADAMTS-5 (0.16–20 nM) (H). Bound protein was detected using M2 anti-FLAG primary antibody and a horseradish peroxidase-coupled secondary antibody.
(I) TIMP-3 (0.5 nM) and ADAMTS-5 (0.5 nM) were incubated (1 hr, 37°C) with various concentrations (10–2000 nM disaccharide) of dp36 heparin (untreated) or glycol-split dp36 heparin. The residual activity against the fluorescent peptide substrate was determined.