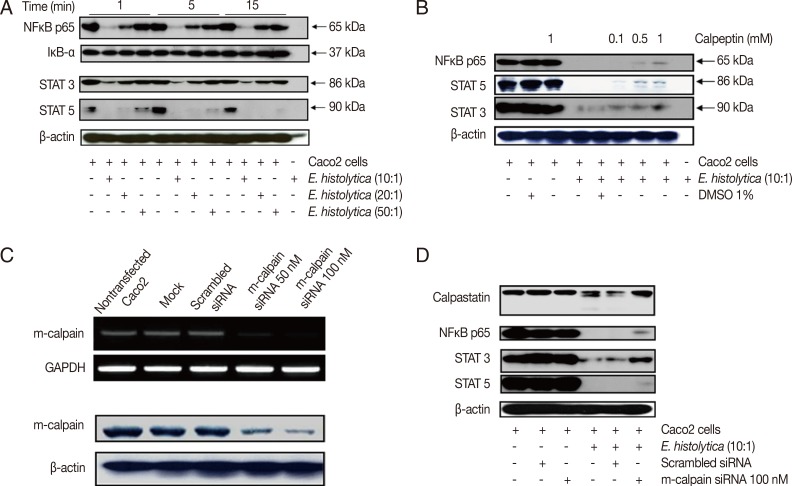
Fig. 2.
Incubation with E. histolytica induces degradation of NF-κB and STATs, but not IκB-α, in Caco-2 cells in a calpain-dependent manner. (A) Caco-2 cells (1×106/sample) were incubated for 1-15 min at 37℃, either with or without E. histolytica at ratios of 10:1, 20:1, or 50:1 (Caco-2 cells to E. histolytica). After incubation, proteins in whole cell lysates were subjected to SDS-PAGE and subsequently Western blotted with anti-NF-κB (p65), anti-IκB-α, anti-STAT3, and anti-STAT5 Abs. β-actin was used as a loading control. (B) Caco-2 cells (1×106/sample), pretreated either with or without calpeptin, were incubated for 15 min at 37℃ in either the absence or presence of E. histolytica (1×105/sample) in a CO2 incubator. After incubation, proteins in whole cell lysa tes were subjected to SDS-PAGE and Western blotted with anti-NF-κB (p65), anti-STAT3, and anti-STAT5 Abs. β-actin was used as a loading control. (C) Downregulation of both mRNA expression levels and protein levels upon transfection of m-calpain siRNA into Caco-2 cells. At 72 hr post-transfection, cDNA and proteins in whole cell lysates from Caco-2 cells transfected with either vehicle alone (mock), scrambled siRNA (negative control), m-calpain siRNA, or nothing (nontransfected) were subjected to RT-PCR and immunoblotting with anti-m-calpain Abs, respectively. β-actin was used as a loading control. (D) At 72 hr post-transfection, proteins in whole cell lysates from Caco-2 cells transfected either with nothing (nontransfected), scrambled siRNA, or m-calpain siRNA, and co-incubated either with or without Entamoeba histolytica, were subjected to SDS-PAGE and subsequently probed with anti-calpastatin, anti-NF-κB (p65), anti-STAT3, and anti-STAT5 Abs. β-actin was used as a loading control. Figures are representative of three independent experiments, each showing similar results.