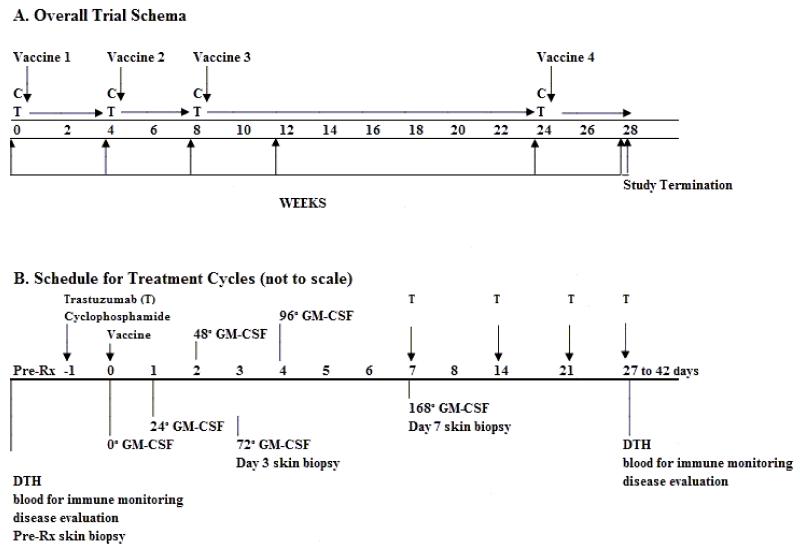
Figure 2. Study schema.
Patients received up to four vaccination cycles. Cyclophosphamide (CY) was given on day −1, and vaccine on day 0. Trastuzumab (T) was given weekly during active vaccine cycles, and timed to coincide with CY administration on day −1. During the interim period between cycles 3 and 4, trastuzumab could be given weekly or every 3 weeks as per the standard of care. GM-CSF levels and immunity were measured as indicated. CY=cyclophosphamide; T=trastuzumab; DTH=delayed-type hypersensitivity to HER-2-derived peptides. Skin biopsies on days +3 and +7 were from the largest vaccine site.