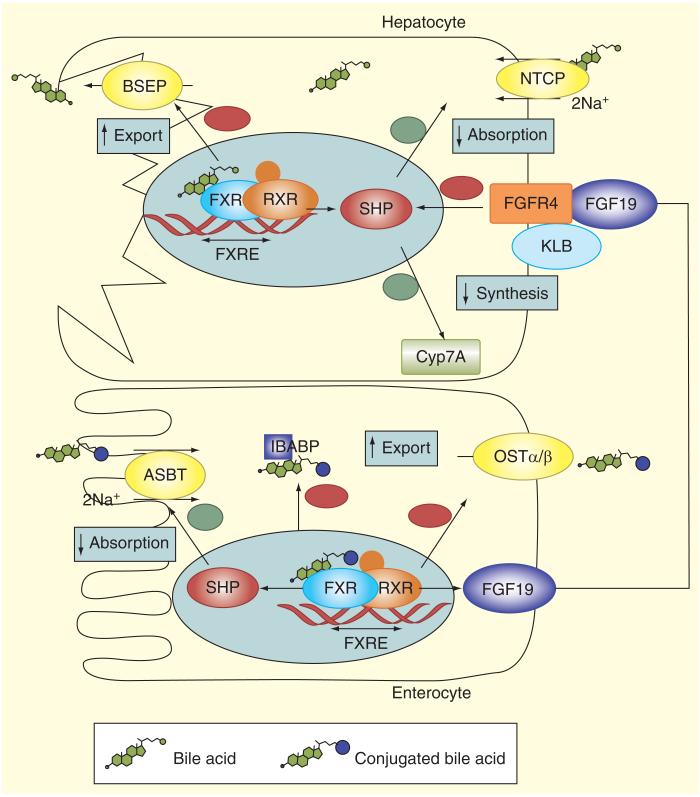
Figure 2. Ileal feedback regulation of bile acid synthesis in hepatocytes.
Intestinal BA uptake has direct and indirect impact on hepatic BA homeostasis. FXR is expressed in ileal enterocytes and hepatocytes. BAs are agonists of the FXR, which modulates gene transcription acting in concert with another nuclear receptor, the retinoid X receptor α (RXRα). BAs exit the enterocyte via the OSTα/β transporter. Sensing of the enterocyte BA pool by FXR affects the liver by way of the endocrine factor FGF19. FGF19 is released to the portal circulation, binds to FGF receptor 4 in hepatocytes, and in a manner that involves biochemical interaction with klotho β, results in downregulation of CYP7A1. This results in inhibition of the classical BA synthetic pathway, both by SHP induction and possibly other pathways. In hepatocytes FXR increases expression of the BSEP and downregulates the expression of NTCP and CYP7A. Since NTCP and BSEP mediate BA uptake from blood and export to bile in hepatocytes and CYP7A catalyzes the limitingrate step in the classical BA biosynthetic pathway, this leads to reduced BA uptake, decreased synthesis and enhanced export to bile.
BA: Bile acid; BSEP: Bile salt export pump; FXR: Farnesoid X receptor; NICP: Na+/taurocholate co-transporting polypeptide transporter; SHP: Small heterodimer partner.
Reproduced with permission [5].