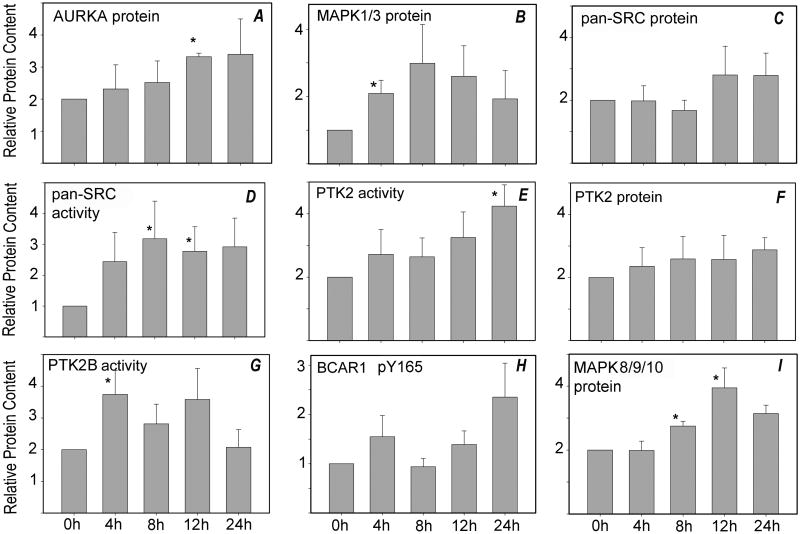
Fig. 2. Western blot analysis of oocytes aged in vitro.
Oocytes were cultured for periods of up to 24 hr (x-axis), then solubilized in SDS-containing sample buffer. Samples (each from a different pool of females) containing 3-5 oocytes were analyzed by SDS-PAGE, as described in ‘Materials and Methods’, and transferred to nylon membranes for Western blot analysis. Blots were probed with antibodies to (A) anti-AURKA (4 samples), (B) anti-MAPK1/3 (6 samples), (C) anti-pan-SRC (7 samples), (D) activated pan-SRC-family kinases (dephosphorylated-Y528; 6-9 samples), (E) anti-PTK2 phospho-Y576 (5 samples), (F) anti-PTK2 protein (4 samples), (G) anti-PTK2B phospho-Y579 (7 samples), (H) anti-BCAR1 phospho-Y165 (6 samples), or (I) anti-MAPK8/9/10 protein (6 samples). Each sample was also probed with anti-GAPDH as a loading control. The bound antibody was detected and quantified as described in ‘Materials and Methods’. Values represent the mean quantity of kinase protein corrected for GAPDH content, which are expressed relative to that present at 0 hr of culture (arbitrarily set as 1.0 on the y–axis). Error bars indicate standard error of the mean. Asterisks (*) indicates that the value was significantly different from 0 hr control (P<0.05).