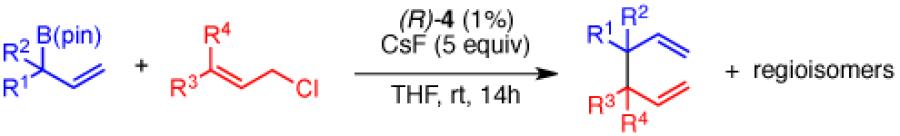
Table 3.
Substrate Scope for Congested Couplingsa
| |||||
|---|---|---|---|---|---|
| entry | allyl boronate |
product | yield (%) |
regio | er |
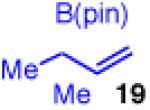
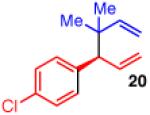
| 1 |
|
|
94 | >20:1 | 98:2 |
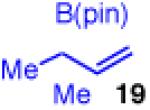
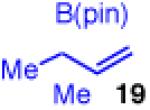
| 2 |
|
|
90 | >20:1 | 98:2 |
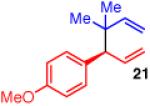
| 3 |
|
|
92 | 5:1 | 96:4 |
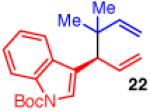
| 4 |
|
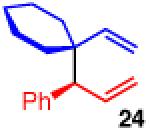
|
85 | >20:1 | 97:3 |
| 5 |
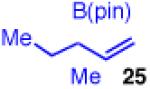
|
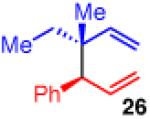
|
96 | >20:1 | 98:2 (2:1 dr) |
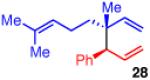
| 6b |
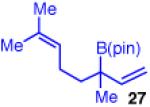
|
|
92 | >20:1 | 98:2 (3:1 dr) |
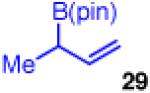
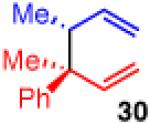
| 7c |
|
|
74 | 5:1 | >99:1 (4:1 dr) |
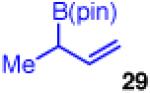
| 8d |
|
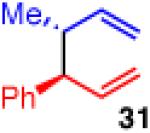
|
90 | >20:1 | 99:1 er (3:1 dr) |
| 9 |
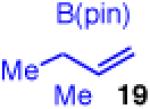
|
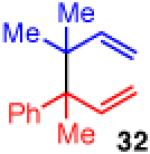
|
<5 | - | - |
| 10 |
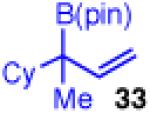
|
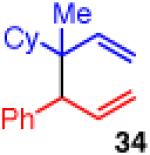
|
<5 | - | - |
| 11e |
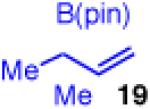
|
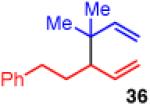
|
<5 | - | - |
Unless otherwise noted reactions were run at 0.5 M concentration of the electrophile with 1.2 equiv of the allylboron reagent. Yield refers to isolated yield of purified material. Enantiomer ratios were determined by GC or SFC analysis on a chiral stationary phase. Diastereomer ratios were determined by 1H NMR analysis. All yield, er and dr values reported are the average of two or more experiments.
Reaction run with 10 equiv CsF for 24 h.
Reaction run in 10:1 THF/H2O.
Reaction run at 60 °C, 0.1% catalyst loading with 0.05 equiv CsF for 4 h.
Reaction run in 10:1 THF/H2O with 1% (S,S)-QuinoxP*PdCl2 in place of (R)−4.
