Abstract
Cullen's sign, ecchymosis of the subcutaneous periumbilical tissue often described in association with non-malignant conditions such as ruptured ectopic pregnancy or acute pancreatitis, has been reported in malignancies involving the abdomen. In melanoma, hematoma-like metastasis has been observed and can resolve with an effective therapy. We observed resolution of Cullen's sign (probably hematoma-like metastasis) in a patient with metastatic melanoma. The patient was participating in a phase I clinical trial and treated with TH-302, a hypoxia-activated prodrug. After 2 months on study, complete resolution of Cullen's sign resolved in concert with extracranial response in lung, liver, and lymph node metastases. Based on the dramatic extracranial response to this investigational agent, additional patients with metastatic melanoma without evidence of brain metastasis were treated on study with TH-302.
Key words: Cullen's sign, TH-302, melanoma, hypoxia-activated prodrug.
Introduction
Cullen's sign, ecchymosis of the subcutaneous periumbilical tissue was often described in association with ruptured ectopic pregnancy or acute pancreatitis until 1922, when Stemnberg described its association with malignancy (adenocarcinoma of the liver).1 Since then, Cullen's sign associated with other malignancies involving the abdomen have been reported. In melanoma, hematoma-like metastasis2–5 has been observed and can resolve with an effective therapy.5 Hematoma-like metastases have not been reported to involve the periumbilical region, masquerading as Cullen's sign. This case report details the treatment course of a patient with metastatic melanoma with resolution of Cullen's sign (probably hematoma-like metastasis) with tumor response to TH-302, a hypoxia-activated prodrug, in a phase I clinical trial,6 leading to further investigation of TH-302 in patients with metastatic melanoma.7
Case Report
A 74-year-old Caucasian man presented to our clinic with metastatic melanoma (MM) involving the lung and liver. Seventeen months prior, he was diagnosed with superficial spreading melanoma on his upper back, Clark level IV, Breslow thickness 1.71 mm with four mitotic figures per high power field. No lymphangitic or perineural invasion was identified. He underwent wide excision with margins of 1 cm. Subsequently, on surveillance CT scans, lung and liver masses suspicious for metastases were identified. CT-guided biopsy of a lung mass confirmed metastatic disease and DTIC was initiated. Initial scans showed a favorable PET/CT response. Three months later, the patient complained of epigastric discomfort and pain and CT scans, a few weeks before our evaluation, demonstrated disease progression.
Other past medical history was unremarkable except for remote history of early-stage prostate cancer resected approximately eight years prior, medication controlled hypertension, and hearing aid use. His only symptoms were pain with deep inspiration and intermittent pain in the right flank. His Karnofsky Performance Status was 90.
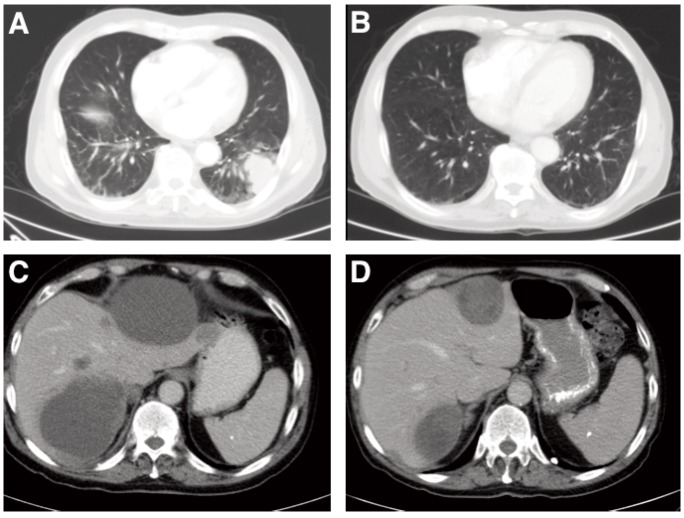
Physical examination was significant for healed surgical scar on the upper back from melanoma removal, firm abdomen and liver palpated 4 cm below the costophrenic margin without splenomegaly, and asymptomatic periumbilical ecchymosis, consistent with Cullen's sign (Figure 1A). Neurological exam was only significant for bilateral hearing loss. Laboratory evaluation revealed an elevated lactate dehydrogenase (LDH) of 1,004 IU/L (ULN = 500 IU/L) and absence of coagulation abnormalities. After signed informed consent, patient was screened and enrolled on the TH-302 phase I trial6 at a dose of 670 mg/m2 i.v. weekly × 3 every 28 days. On CT imaging prior to dosing, a 3.8 cm lobulated, left lower lung mass (Figure 2A), 2.7 cm paracaval lymph node, and multiple liver masses (largest was 13.3 cm) were identified (Figure 2C). Prior to his second weekly dose, asymptomatic blistering and scabbing of skin on the medial surface of the right arm near the i.v. infusion site was observed. He noted a decrease in epigastric discomfort. The second and third doses were delivered in the opposite arm. In his off-dosing week, he reported to his primary oncologist with bilateral upper extremity swelling and was diagnosed with superficial thrombophlebitis after Doppler ultrasound. No pulmonary embolism was identified on CT angiography. He was started on low molecular weight heparin (LMWH) and coumadin. At cycle 2 week 1 dosing, mild-moderate fatigue, dry non-productive cough, and petechial rash on his lower extremities were observed. Physical exam revealed a softer abdomen and significantly smaller liver, now palpable to 2 cm below the costophrenic margin, with less prominent periumbilical ecchymosis. He also had mild petechial rash of the lower extremities and perianal redness, causing minimal discomfort. Bilateral extremities had blistering and healing scabs. No new neurological findings were identified.
Figure 1.
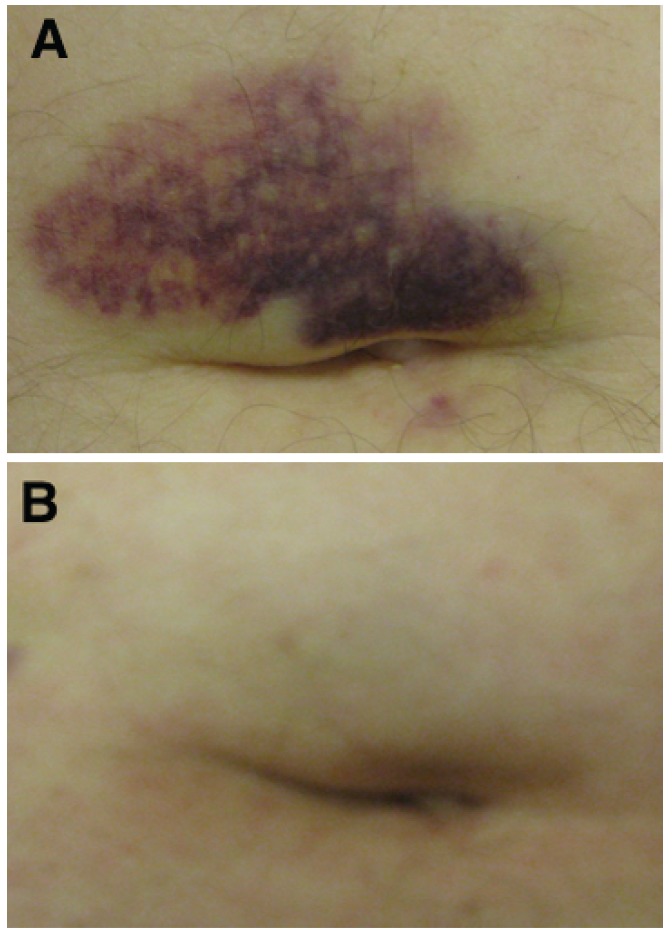
A)Photograph of Cullen's sign at baseline. B) Photograph of Cullen's sign prior to cycle 3.
Figure 2.
A) Computed tomography scan image depicting lung mass at baseline. B) Computed tomography scan image depicting lung mass prior to cycle 3. C) Computed tomography scan image depicting representative liver metastases at baseline. D) Computed tomography scan image depicting representative liver metastases prior to cycle 3.
Evaluation of his outside CT angiogram revealed improvements in target lesion diameters by RECIST criteria (22% decrease).8 After insertion of a central line, cycle 2 was initiated with heat packs and NSAIDs for superficial thrombophlebitis per guidelines.9
On cycle 2 week 2 visit, patient reported increased pain, swelling, and palpable cord in his right arm for three days. He also noted an ulceration on his left arm near the site of prior i.v. insertion, as well as, mild rectal pain and bright red blood after wiping. Physical exam revealed continued decrease in palpable liver and near resolution of periumbilical ecchymosis. A palpable firm and tender cord was noted in the right forearm, and rectal examination revealed no ulceration, hemorrhoid, mass, or gross blood. Doppler ultrasound of the upper extremities revealed deep vein thrombosis in the left basilic vein. LMWH was re-initiated. LDH was decreased to 213 IU/L. Cycle 2 weeks 2 and 3 dosing were delivered uneventfully.
Prior to cycle 3, a protocol scheduled CT demonstrated partial response by RECIST criteria8 (decrease in diameters of target lesions of 53.4%)(Figures 2B and 2D). At cycle 3 week 1 dosing no new symptoms or physical findings were noted, Cullen's sign resolved (Figure 1B). LDH was now down to 169 IU/L.
Unfortunately, 6 days later he developed right-sided neurological symptoms including weakness, uncontrollable twitching of his right arm, possible seizure and post-ictal confusion. MRI of the brain revealed multiple enhancing lesions in the left frontal and parietal lobes (largest measuring 3.5 cm). The patient had seizures refractory to medical therapy before whole brain radiotherapy could be initiated. He became unresponsive and per advanced directives and discussion with the family, comfort measures were initiated. He was transferred to inpatient hospice and died shortly thereafter.
Discussion
This case report highlights a rare event of MM associated Cullen's sign. Even more intriguing was the resolution of periumbilical ecchymosis after several treatments of TH-302, a hypoxia-activated prodrug, in concert with significant extracranial tumor regression. Up until the time the seizures began, there was no new neurological finding prior to start of the study or during the treatment. It is probable that brain metastasis were present but unrealized at the initiation of treatment and then progressed, and less likely that new brain metastasis developed while the patient's extracranial sites were significantly decreasing in size. Based on the dramatic extracranial response, additional patients with MM have been treated with TH-302 at the slightly lower MTD dose of 575 mg/m2.7
Acknowledgment:
we are indebted to the patient and his family, providers, and staff and Dr. John Curd for their contributions.
References
- 1.Mabin TA, Gelfand M. Cullen's sign, a feature in liver disease. BMJ. 1974;1:493–4. doi: 10.1136/bmj.1.5906.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torok L, Kirschner A, Ócsai H, Olasz K. Hematoma-like metastasis in melanoma. J Am Acad Dermatol. 2003;49:912–3. doi: 10.1016/s0190-9622(03)00463-8. [DOI] [PubMed] [Google Scholar]
- 3.Jouary T, Delaunay M, Taieb A. Case letters: hematoma-like metastases. J Am Acad Dermatol. 2006;55:1106–7. doi: 10.1016/j.jaad.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Connolly CM, Soldin M, Cooper AC, Dawson A. Metastatic malignant melanoma presenting with a bruise. Br J Plast Surg. 2003;56:76–76. doi: 10.1016/s0007-1226(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 5.Dehart WK, Gilliam AC, Lu KQ, Brell J. A rare case of melanoma recurring as subcutaneous metastatic melanoma with overlying ecchymoses. Arch Dermatol. 2008;144:561–2. doi: 10.1001/archderm.144.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Weiss GJ, Infante JR, Chiorean EG, et al. Phase 1 Study of the Safety, Tolerability and Pharmacokinetics of TH-302, a Hypoxia-Activated Prodrug, in Patients with Advanced Solid Malignancies. Clin Cancer Res. 2011 Mar 17; doi: 10.1158/1078-0432.CCR-10-3425. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Curd JG, Infante R, Weber R, et al. Initial Clinical Observations Regarding TH-302, a Tumor Selective Hypoxia-Activated Prodrug, in Metastic Melanoma. Melanoma Perspective XIII meeting. 2009 Available from: http://www.thresholdpharm.com/sec/posters.
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]