Fig. 4.
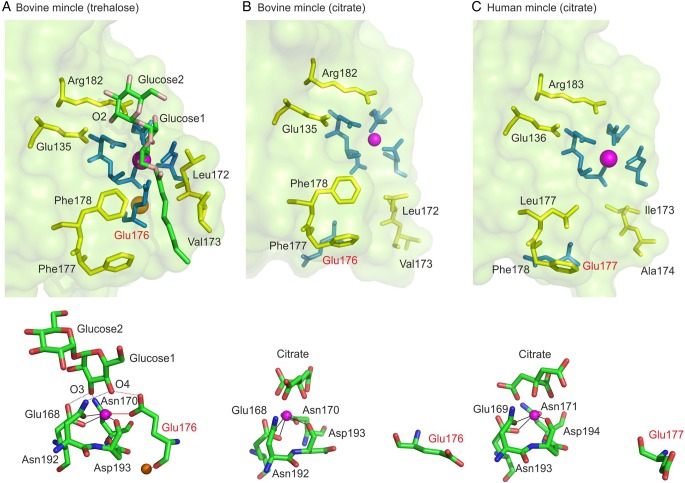
Structures of CRDs from mincle. Upper panels show the overall binding site, whereas lower panels show the residues that ligate to the conserved Ca2+. (A) Structure of bovine mincle with trehalose bound (PDB 4kzv). Side chains highlighted in green form the primary sugar-binding site by ligating to the conserved Ca2+, shown in magenta, and by making hydrogen bonds with 3- and 4-OH groups in the first glucose residue in trehalose. Side chains that form hydrogen bonds with the 2-OH group of the second glucose residue are highlighted in yellow, as are side chains that are proposed to form a binding site for the acyl chain attached to trehalose. A secondary cation near the sugar-binding site is shown in orange. This cation is Na+ in the previously reported structure of trehalose bound to bovine mincle (Feinberg et al. 2013), but similar crystals in which Ca2+ is present at this site have been analyzed subsequently (Hadar Feinberg, Kurt Drickamer and William I. Weis, unpublished observations). (B) Structure of bovine mincle with citrate bound (PDB 4kzw). Residues from the binding site in (A) are shown using the same color scheme. Key differences in the structure result from displacement of loops of protein containing Ca2+ ligand Glu135 and residues Leu172 and Val173, which form one side of the hydrophobic groove in the ligand-bound conformation. Citrate is omitted from the top panel for clarity. (C) Structure of human mincle (PDB 3WH2). Highlighting of residues is as in (A). The loops of protein containing potential Ca2+ ligand Glu136 and residues Ile173 and Ala174, corresponding to residues Glu135, Leu172 and Val173 in bovine mincle, show the conformation very similar to those seen in (B). Citrate is omitted from the top panel for clarity. This figure was created using PyMol.