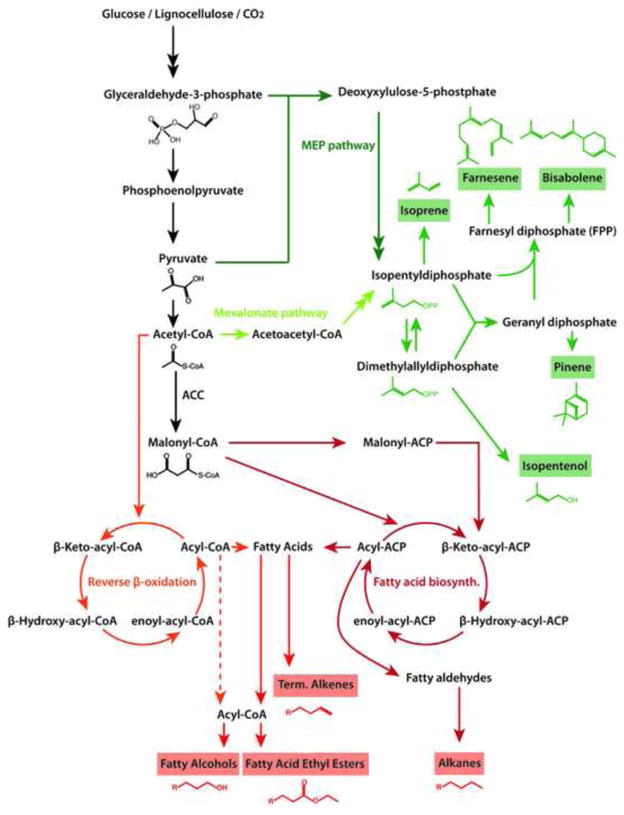
Figure 3. Biosynthesis of isoprenoid and fatty acid biofuels.
Isoprenoids (green) are produced by successive condensation of the 5-carbon precursors isopentenyl-pyrophosphate (IPP) and dimethyl-allyl pyrophosphate (DMAP), which are isomers of each other. IPP and DMAP are synthesized by either the mevalonate pathway (found in the cytosol of plants) or the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway (also known as the non-mevalonate or 1-deoxy-D-xylulose-5-phosphate (DXP) pathway), which is native to E. coli and cyanobacteria. Fatty acids (FAs, red) are synthesized from malonyl-CoA by multi-enzyme fatty acid synthases. Malonyl-CoA is made by acetyl-CoA carboxylase (ACC), the rate-limiting and committed first step in FA biosynthesis. The growing FA chains are attached to acyl-carrier proteins (ACP). Thioesterases cleave fatty acids off the ACP. Reverse β-oxidation offers an alternative pathway that uses CoA as a carrier molecule and acetyl-CoA to elongate the growing chain instead of malonyl-CoA. Double-headed arrows represent multiple steps.