Figure 1.
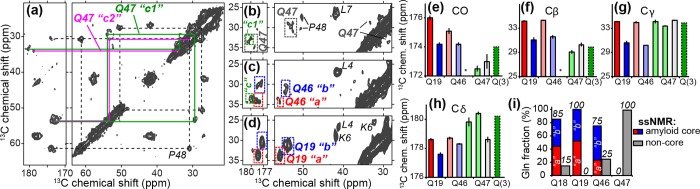
13C chemical shift assignments via 2D MAS ssNMR spectra. (a) Aliphatic-carbonyl (left) and intra-aliphatic (right) spectral regions from an 8 ms DARR 13C–13C 2D spectrum on LQP-labeled httNTQ30P10K2 fibrils at 275 K. (b–d) Comparison of individual Gln residues in different parts of the polyQ domain: (b) Q47 (15 ms DARR, 9.8 kHz MAS), (c) Q46 (8 ms DARR, 10 kHz MAS), and (d) Q19 (25 ms DARR, 13 kHz MAS, data from ref (27)). In these spectra, color-coded lines, boxes, and quoted letters (“a”, “b”, etc.) mark the Gln conformers discussed in the text. In panels b–d, peaks from labeled residues L4, K6, L7, and P48 are indicated. (e) Backbone and (f–h) side-chain 13C chemical shifts for Gln in httNTQ30P10K2 fibrils. Red/blue color coding indicates conformers “a” and “b” (see refs (27) and (48)). Newly observed resonances for Q46 and Q47 are shown with paler color to indicate the conformers, where applicable. Green conformers for Q46 and Q47 resemble a “Q(3)” conformer reported in polyQ fibrils,64 with a Cδ shift near 180 ppm. Missing resonances are indicated with asterisks. (i) Fractions of residues Q18, Q19, Q46, and Q47 that feature the amyloid core signature, estimated from ssNMR 2D peak volumes.
