Figure 2.
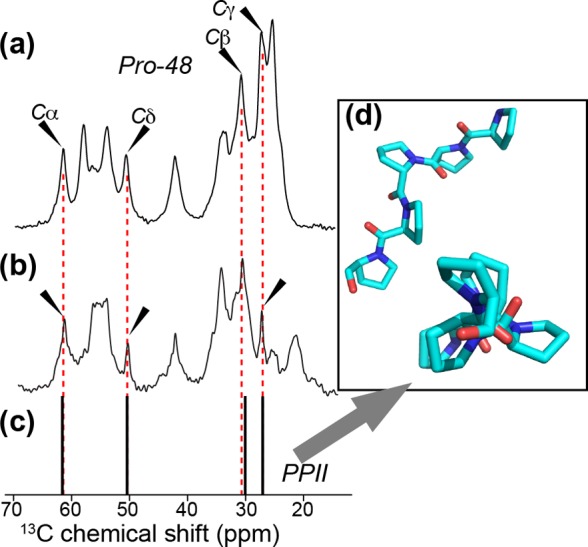
13C chemical shifts for Pro in httNTQ30P10K2 fibrils. (a) 1H–13C 1D CP MAS spectrum of LQP-labeled fibrils at 9.8 kHz MAS, showing the peaks due to the first Pro (P48, black arrows). (b) Natural abundance Pro signals (black arrows) in unlabeled httNTQ30P10K2 fibrils at 10 kHz MAS.27 Unmarked peaks are due to other residues, either isotopically enriched (in panel a) or at natural abundance. Panel (b) adapted with permission from ref (27). Copyright 2011 American Chemical Society. (c) Chemical shifts of Pro in an example of PPII-structured oligoPro (shown in panel d), from previously published solution NMR on a chimera of the peptide APSYSPPPPP and α-spectrin SH3.62
