Figure 7.
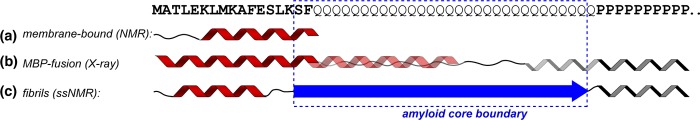
Secondary structure of htt N-terminal fragments under differing conditions. (a) NMR studies have shown extensive α-helicity in isolated httNT upon interacting with a lipid bilayer.77 (b) In crystals formed from a MBP fusion construct, httNT is fully α-helical, followed by partial α-helicity in the N-terminal part of the polyQ domain.33 The oligoPro segment forms a PPII helix, with extended or PPII structure for several preceding Gln residues. (c) Mature fibrils feature a relatively well-defined β-sheet amyloid core (dashed blue box; see also Figure 8), formed at the expense of any α-helical or PPII structure within the polyQ. A short α-helix in httNT helps stabilize the fibrils via molten-globule-like assemblies that may be inherited from non-β oligomers (see the text).
