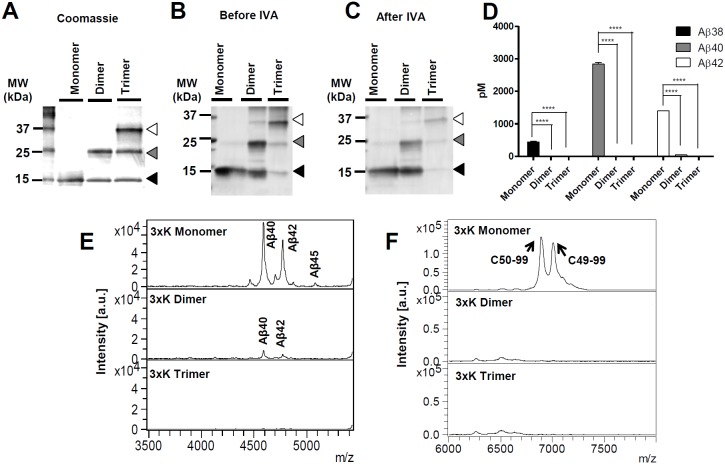
Figure 3. Recombinant 3xK-C100Flag monomer increased longer Aβ peptides.
(A) The purified monomer, dimer, and trimer of recombinant 3xK-C100Flag were loaded on a SDS-PAGE gel and identified from the gel stained by Coomassie blue. The size of the monomer was at ∼12 kDa (black arrow), the dimer was at ∼24 kDa (grey arrow), and the trimer was at ∼36 kDa (open arrow). (B–C) Western blot of the purified monomer, dimer, and trimer 3xK-C100Flag substrates before/after IVA did not show significant changes in relative amounts of the various multimers. (D) Aβ ELISAs showed that Aβ production measured from 3xK-C100Flag dimer substrate is significantly reduced compared to that from 3xK-C100Flag monomer, but that there is no evidence for major shifts in Aβ ratios (E). Aβ profiles generated from IP/MS of the media show that for 3xK-C100Flag there is increased relative production of longer Aβ isoforms compared to WT substrate (see Figure 2). Molecular mass (m/z) of monomeric 3xK-C100Flag for Aβ40 is 4588.43 (calculated m/z: 4589.27), for Aβ42 is 4773.07 (calculated m/z: 4773.51), and for Aβ45 is 5084.52 (calculated m/z: 5086.90). 3xK-C100Flag dimer and trimer showed decreased Aβ levels compared to the monomer. Molecular mass of dimeric 3xK-C100Flag for Aβ40 is 4587.48 (calculated m/z: 4589.27) and for Aβ42 is 4769.235 (calculated m/z: 4773.51). (F) AICD profiles show that C49-99 and C50-99 are the dominant peaks for 3xK-C100Flag monomer and that this profile is similar to that observed for WT substrate. Molecular mass (m/z) of monomeric 3xK-C100Flag for AICD50-99 is 6915.460 (calculated m/z: 6905.66), for AICD49-99 is 7027.141 (calculated m/z: 7018.82). AICD production from 3xK-C100Flag dimer and trimer is markedly decreased in comparison to monomer. This experiment was performed twice with duplicates. Results were analyzed by two-way analysis of variance (ANOVA) followed by bonferroni post hoc testing (****p<0.0001).