Abstract
Objective: This study aims to compare the difference and the change trend of Muscle Architecture Parameters (MAP) between spastic and normal muscle tone patients after stroke, and analysis the application and value of Muscle Architecture Parameters in evaluating spasticity after stroke. Methods: 41 stroke patients were divided into spastic group (26 cases), normal muscle tone control group (15 cases). Modified Ashworth Scale (MAS) was applied in both groups for assessing muscle tone of lower limbs (no influence, contralateral). Muscle architectural parameters of ultrasound measurement include muscle thickness, fascicle length and pennation angle. The difference of three muscle architectural parameters between the affected side and the contralateral side in spastic group and the difference of MAS and three muscle architectural parameters between spastic group and normal control group were compared. Results: MAS score, Pennation Angle (PA) and Muscular Thickness (MT) value of MAP in spastic group were significantly higher than the control group, Fascicle length (FL) is significantly lower than the control group (P < 0.05). In spastic group, MAS score, PA and MT value of MAP of affected side muscle was substantially higher than that of contralateral, FL value significantly lower than that of contralateral (P < 0.05). There was positive correlation between MAS and PA and MT but was negative correlation between MAS and FL, rank correlation coefficient test was statistical significant (p < 0.05). Logistic multivariate regression analysis showed that spasticity can lead PA and FL to change (p < 0.05), there is no clear correlation between MT and spasticity occurs (P > 0.05). Conclusion: MAP has a better sensitivity in evaluating muscle tone between spastic patients and non-spastic patients, and degrees of spasticity have a clear corresponding exponential relationship to MAP. Combing MAS and MAP can assess muscle tone more objectively and accurately because subtle changes can be observed by testing values of architecture parameters that compensating for the shortcomings of MAS in reliability and validity. Thus it is helpful for guiding clinical antispastic practice.
Keywords: Pennation angle, muscular thickness, fascicle length, stroke, spasicity
Introduction
Stroke is the leading cause of death and the principle cause of long-term neurological disability worldwide [1-3]. Approximately two thirds of patients with stroke have profoundly impaired motor function [4]. Reduced upper limb function leads to significant disability that affects daily living and increases the burden on these patients and their families [5,6].
Accurate assessment and objective observation of muscle tone are important basis for clinician to make treatment plan. At present, semi-quantitative grading scale is mainly used in evaluation and comparison of the degree of muscle tone. Modified Ashworth Scale (MAS) was most widely used for assessing spasticity in clinical practice and research. MAS for grading spasticity are based on measuring resistance during passive soft-tissue stretching and passive range of motion under the resistance.
Other types of scale such as the Penn scale testing frequency of limb spasm and Tardieu scale quantifies muscle spasticity by assessing the response of the muscle to specified stretch velocities and so on. Although the defined standards are different, they all belong to semi-quantitative grading scale. These methods used to check muscle tone occurs are easily influenced by rater’s experience, subjective judgment capacity and understanding of scale standards etc. Thus it may lead to deviation of the test results.
So, it is necessary to explore more objective and more accurate quantitative evaluation methods of muscle tone, such as EMG measurement, ultrasonic image measurement of muscle, mechanical analysis of muscle fibers. In recent years, study of MAP in spastic patients primarily focus on parameter change observation after spasticity occur, but further correlation analysis between parameters change trends under different muscle tone has not been done.
In this study, we measured the parameters in limbs of spastic and normal muscle tone patients, analyzed the effect of different architecture parameters change on the spasticity and screened affecting architecture parameters to explore the objective evaluation method of muscle tone.
Methods
Patients
Patients were recruited from outpatients and inpatients in Department of Rehabilitation Medicine and Neurology in the Xuanwu Hospital of Capital Medical University, since January 2013-June 2013, they were divided into 2 groups: spastic group and control group. There were 26 patients (male 18, female 8) with spasticity post stroke in spastic group, 17 cases of cerebral infarction, 9 cases of cerebral hemorrhage (median age was 54.64 ± 15.68 years, average time of onset was 4.38 ± 2.77 month). Inclusion criteria for this group: (1) the first stroke in the course < 12 months. (2) Head CT/MRI showed solitary lesion of infarction or hemorrhage one side of brain. (3) The muscle tone in the affected limb, MAS: 1~4, in contralateral normal limb, MAS: 0. (4) Limb Motor dysfunction: 0 < FMA < 85, ADL, impaired self-care ability: 0 < BI < 85. (5) Conscious, orientation, memory, comprehension is normal, examination cooperation. There were 15 stroke patients (male 11, female 4) with normal muscle tone in control group, 11 patients were diagnosed with cerebral infarction and 4 patients with cerebral hemorrhage, median age was 51.80 ± 9.26 years, the time since stroke was 3.73 ± 1.75 months. Inclusion criteria were the same as the spastic group in addition to MAS of the muscle tone in the affected limb 0. All patients signed an informed consent form. This study was approved by the Ethics Committee of the Xuanwu Hospital of Capital Medical University.
MAS assessment
Patients of spastic group and normal control group accepted MAS assessment. Evaluation of muscle tone with MAS were performed by a 3-year experienced rehabilitation physician.
Collection of ultrasound images
Muscle ultrasound image were acquired with PHILIPS IU-22 ultrasound equipment. Ultrasound probe is L12-5-flat trigger ultrasonic detection tube. Embedded CMSP measure software of PHILIPS IU-22 was used to measure the image. Ultrasound images of subject were collected by the same sonographer with over 5-year professional experiences. Subject lied prone on the examination bed quietly, exposing bilateral legs, with feet down on her bedside and keeping ankle in neutral position. Examiner exerted a resistance at the foot of the subject and asked the subjects to do ankle plantar flexion at the same time to reveal the gastrocnemius profile completely. Plantar flexors located at the midpoint position of the connecting line between the calcaneus and central popliteal fossa then relax.
PHILIPS IU-22 type Ultrasonic Tester is adjusted and check frequency is set to 25-35 Hz, check type using 2D, checked pattern using RS, gray type using M2, ECHO collection rate is set to 70%-85%, ultrasonic spectrum value C = 56, probes generator L12-5 using soft tissue muscle-specific checking model Vasc Ven. PA, MT and FL are measured using built-in CMSP software of PHILIPS IU-22 Tester.
Data analysis
The analysis was performed with statistical software (SPSS 17.0). To assess between group PA, MT, FL, independent-samples t-test was performed to assess PA, MT and FL in spastic side and normal side of spastic group, paired t-test was performed; to assess the relevance between PA, MT, FL and MAS in both groups, spearman coefficients were performed; to assess the relevance between PA, MT, FL and spasticity in both groups, logistic multiple regression was performed.
Results
Baseline characteristics of the 2 groups
Independent t tests and chi-square test were used to compare baseline characteristics of the 2 groups. The age, sex, and disease, time since stroke between the two groups had no significant difference (P > 0.05) (Table 1).
Table 1.
Baseline characteristics of the 2 groups
| Age (years) | Gender (male/female) | Disease (infarction/hemorrhage) | Time since stroke (months) | |
|---|---|---|---|---|
| Spastic group | 54.64 ± 15.68 | 18/8 | 17/9 | 4.38 ± 2.77 |
| Control group | 51.80 ± 9.26 | 11/4 | 11/4 | 3.73 ± 1.75 |
| F/X2 | 0.65 | 0.07 | 0.27 | 0.81 |
| P | 0.52 | 0.78 | 0.59 | 0.41 |
MAS scores in spastic group and control group
The MAS scores in spastic group is (2.38 ± 0.85), the MAS scores in control group is (0.00 ± 0.00). There was significant difference in MAS between two groups (P < 0.05).
PA, MT and FL in spastic group and control group
PA of spastic group was 25.09 ± 5.65°, PA of control group was 15.43 ± 3.60°; MT of spastic group was 14.09 ± 2.71 mm, MT of control group was 10.86 ± 1.44 mm; FL of spastic group was 35.82 ± 4.08 mm, FL of control group was 40.04 ± 3.88 mm. There were significant differences between two groups (P < 0.05) (Table 2).
Table 2.
PA, MT and FL in spastic group and control group
| PA (degree) | MT (mm) | FL (mm) | |
|---|---|---|---|
| Spastic group | 25.09 ± 5.65 | 14.09 ± 2.71 | 35.82 ± 4.80 |
| Control group | 15.43 ± 3.60 | 10.86 ± 1.44 | 40.04 ± 3.88 |
| t | 5.94 | 4.25 | -2.89 |
| P | 0.00 | 0.00 | 0.00 |
MAS in spastic side and normal side of spastic group
The MAS scores in spastic side is (2.38 ± 0.85), the MAS scores in normal side is (0.00 ± 0.00). There was significant difference in MAS between two sides (P < 0.05).
PA, MT and FL in spastic side and normal side of spastic group
There were significant differences in PA (spastic side (25.09 ± 5.65°), normal side (16.45 ± 4.90°), P < 0.05), MT (spastic side (14.09 ± 2.71) mm, normal side (9.48 ± 2.36) mm, P < 0.05) and FL (spastic side (35.82 ± 4.08) mm, normal side (40.70 ± 3.25) mm, P < 0.05) between two groups (Table 3, Figure 1).
Table 3.
PA, MT and FL in spastic side and normal side of spastic group
| PA (degree) | MT (mm) | FL (mm) | |
|---|---|---|---|
| Spastic side | 25.09 ± 5.65 | 14.09 ± 2.71 | 35.82 ± 4.80 |
| Normal side | 16.45 ± 4.90 | 9.48 ± 2.36 | 40.70 ± 3.25 |
| t | 5.89 | 6.52 | -4.29 |
| P | 0.00 | 0.00 | 0.00 |
Figure 1.
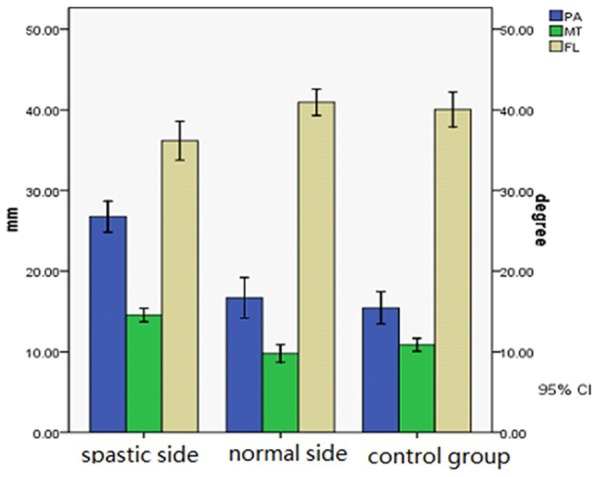
PA, MT and FL in spastic side, normal side of spastic group and control group. There were significant differences in PA, MT and FL between spastic side and normal side of spastic group (P < 0.05) and between spastic group and control group (P < 0.05).
Correlation analysis of muscle architecture parameters PA, MT, FL and degree of muscle spasticity
We use rank conversion to MAS scores because MAS scores are ranked data. Spearman coefficients were used to assess the relevance between PA, MT, FL and MAS. Coefficients of correlation rs were tested. PA was associated with MAS (rs = 0.551, P < 0.05), FL was associated with MAS (rs = 0.615, P < 0.05), MT was associated with MAS (rs = 0.722, P < 0.05) (Table 4, Figure 2).
Table 4.
Correlation between MAS and PA, FL, MT
| rs | P | |
|---|---|---|
| MAS-PA | 0.551 | 0.00 |
| MAS-FL | -0.615 | 0.00 |
| MAS-MT | 0.722 | 0.00 |
Figure 2.
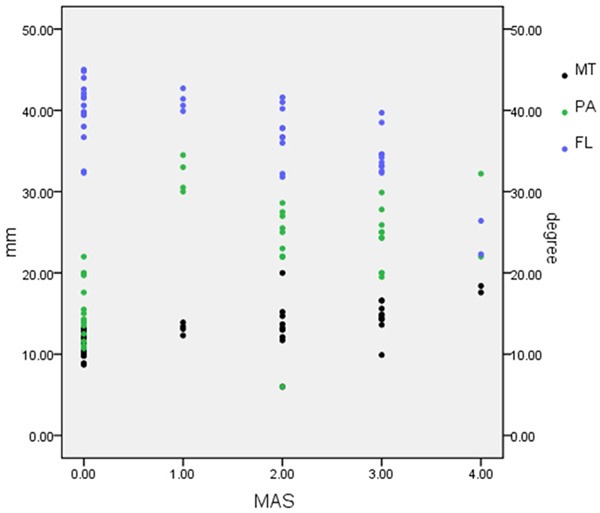
Scatter distribution of different MAS and related PA, FL and MT. MAS has positive correlation with PA and MT, while has negative correlation with FL.
The data distribution type of muscle architecture parameters unmatched the criteria of linear regression, so logistic multiple regression was performed. Defined independent variables as: X1 = PA, X2 = FL, X3 = MT. Defined dependent variables as: Y = spasticity/no spasticity (Y = 0: MAS = 0; Y = 1: MAS ≠ 0). Forward (LR) method was used to filtrate independent variables. Omnibus Tests χ2 = 36.60 (P < 0.05). PA (OR = 1.66, 95% CI = 1.16-2.39) and FL (OR = 0.57, 95% CI = 0.35-1.92) entered regression equation, MT did not enter regression equation (residual error χ2 = 0.02, P = 0.878) (Table 5).
Table 5.
Logistic multiple regression of spasticity and PA, FL, MT
| Dependent variables | B | S.E. | Wald | df | P | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|---|
| PA | 0.51 | 0.18 | 7.76 | 1 | 0.00 | 1.66 | 1.16-2.39 |
| FL | -0.55 | 0.24 | 5.21 | 1 | 0.02 | 0.57 | 0.35-1.92 |
| Constant | 11.67 | 7.28 | 2.56 | 1 | 1.10 | 117487.80 |
B, regression coefficient; S.E., standard error; Wald, Wald value; df, degree of freedom; Exp (B), OR value.
Discussion
The role of modified ashworth scale in assessing muscle tone
The clinical muscle tone classification scale was an important tool in assessing the muscle tone. The majority of scales were based on clinical sign caused by spasticity. For instance, the Penn scale was based on frequency of spasm during a defined period, Tardieu scale was based on reaction density of extremity with three different gravity accelerations, and MAS was based on ROM and torque when spasticity occurs. Among the former three scales, MAS were more widely used in clinical practice than others, which was put forward by Ashworth in 1964 including five grades (from 0 to 4) then added to six grades (added 1+ grade).
The MAS were commonly thought of satisfactory reliability and validity in preliminary evaluation of abnormal muscle tone in clinical practice, which there are lots of advantages such as convenient, simple and easy to master in short period. There are good interrater and intrarater reliability with MAS to assess typical spasticity and normal muscle tone, because there was significant difference between them [4]. Nevertheless some research results demonstrated that there would possible be testing error and results shift when MAS was used to distinguish untypical spasticity and normal muscle tone, and no significant difference between them [5]. The reasons of this phenomenon were related with difference of tester’s clinical experience, personal judgment ability and understanding for assessment criteria. Therefore some recent researches suggested that the criteria of MAS should be modified (modified Ashworth scale, MMAS) to compensate its reliability and validity [7,8] or combining with other objective methods to reduce the error when MAS was used to evaluate muscle tone.
Comparison and analysis for muscular architecture parameters to assess spasticity and normal muscle tone
Biomechanical researches always focus on the muscular architecture parameters in recent 20 years, and some results indicated that some of parameters were tightly related with muscle fibers biomechanic [9]. FL was the most important parameter in the direction paralleling muscular axis when the muscular fiber contracted actively or was stretched passively, which was the first parameters observed in biomechanics research [10]. PA was defined by angle between direction of muscle bundle and direction of muscle axis as muscle tension or relaxation, which reflected trigonometric function of axis torque and its component torque [11]. PA and FL would change following different joint flexion angle when the muscle completes maximal voluntary contraction, the same results could be found when the muscle completed different percentage of maximal voluntary contraction [12]. It could be confirmed that axial torque and these two parameters were correlative. When the muscle was stretched passively, the PA and FL would change with different stretching torque yet, but change regular was different from that of muscle contraction actively [13]. MT was defined by torque in vertical direction of muscle bundle membrane, which reflected the hardness of bundle lateral membrane. Biomechanical study of muscle stretched passively indicated that MT would change under different stretching torque [14]. The above researches proved that the three muscular architecture parameters were influenced together by stretching torque. It was possible to assess the muscle tone by using muscular architecture parameters in clinical practice, because stretching torque and muscle tone are correlative [15-17]. The previous studies only focus on the change tendency of muscular architecture parameters, but our study have analyzed the correlation between spasticity and three parameters, further observed quantitative changes of them [18].
The results show that PA and MT of spasticity group are higher than those of control group and FL is significantly lower than that of the control group. It also illustrates that increased muscle tone or spasticity make muscle fibers produce concentric contractions and stretch torque increasing. Thus, component of torque perpendicular to the muscle fascicles membrane and angle to axial torque increased. The same results are observed between the affected side and the unaffected side of spasticity group. Therefore the sensitivity of muscular architecture parameters reflecting the change of muscular tension was as good as that of MAS when there was significant difference between spasticity and normal muscle tone. Some architecture changes including decreasing of muscular length, increasing of muscular diameter and deviation from axis for muscle bundle can also be found by testing the architecture parameters, while they can not be concluded by testing MAS.
In addition, spearman correlative analysis confirmed this change tendency of muscular architecture parameters. This change regulation of muscular architecture parameters existed whether there was spasticity or not and in the condition of different degree of spasticity. When muscle tone increases, PA and MT decreases and FL increases according to this changing tendency and shifting regulation. Logistic regression analysis also indicated that the muscle tone was affected by some architecture parameters such as FL and PA. The changes of muscular architecture are as follows: shortening of muscular bundle length, increasing of muscular diameter and increasing of angle between axis and muscle bundle.
Conclusion
Spasticity and normal muscle tone can be assessed and distinguished accurately using the muscular architecture parameters. There is positive correlation between MAS and PA, MAS and MT. There is negative correlation between MAS and FL. The possibility of spasticity is affected by FL and PA.
Combing MAS with muscular architecture parameters can evaluate abnormal muscle tone and spasticity in clinical practice, the changes of muscular construction can be observed by testing values of architecture parameters. Therefore comparison and analysis of muscular architecture parameters by ultrasonic testing will assess spasticity objectively in the future practice.
Acknowledgements
This project was supported by The National Natural Science Fund of China (81000637).
Disclosure of conflict of interest
None.
References
- 1.Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: limb and muscle group effect. NeuroRehabilitation. 2008;23:231–237. [PubMed] [Google Scholar]
- 2.Blackburn M, van Vliet P, Mockett SP. Reliability of measurements obtained with the modified Ashworth scale in the lower extremities of people with stroke. Phys Ther. 2002;82:25–34. doi: 10.1093/ptj/82.1.25. [DOI] [PubMed] [Google Scholar]
- 3.Gregson JM, Leathley M, Moore AP, Sharma AK, Smith TL, Watkins CL. Reliability of the Tone Assessment Scale and the modified Ashworth scale as clinical tools for assessing poststroke spasticity. Arch Phys Med Rehabil. 1999;80:1013–1016. doi: 10.1016/s0003-9993(99)90053-9. [DOI] [PubMed] [Google Scholar]
- 4.Waninge A, Rook RA, Dijkhuizen A, Gielen E, van der Schans CP. Feasibility, test-retest reliability, and interrater reliability of the Modified Ashworth Scale and Modified Tardieu Scale in persons with profound intellectual and multiple disabilities. Res Dev Disabil. 2011;32:613–620. doi: 10.1016/j.ridd.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Campanini I, Merlo A, Cavazzuti L. What’s the risk of using the Modified Ashworth Scale (MAS) to assess spasticity at the ankle? Gait & Posture. 2011;33:S18–19. [Google Scholar]
- 6.Ansari NN, Naghdi S, Mashayekhi M, Hasson S, Fakhari Z, Jalaie S. Intra-rater reliability of the Modified Modified Ashworth Scale (MMAS) in the assessment of upper-limb muscle spasticity. NeuroRehabilitation. 2012;31:215–222. doi: 10.3233/NRE-2012-0791. [DOI] [PubMed] [Google Scholar]
- 7.Naghdi S, Ansari NN, Azarnia S, Kazemnejad A. Interrater reliability of the Modified Modified Ashworth Scale (MMAS) for patients with wrist flexor muscle spasticity. Physiother Theory Pract. 2008;24:372–379. doi: 10.1080/09593980802278959. [DOI] [PubMed] [Google Scholar]
- 8.Sköld C, Harms-Ringdahl K, Hultling C, Levi R, Seiger A. Simultaneous Ashworth measurements and electromyographic recordings in tetraplegic patients. Arch Phys Med Rehabil. 1998;79:959–965. doi: 10.1016/s0003-9993(98)90095-8. [DOI] [PubMed] [Google Scholar]
- 9.Barber L, Barrett R, Lichtwark G. Passive muscle mechanical properties of the medial gastrocnemius in young adults with spastic cerebral palsy. J Biomech. 2011;44:2496–2500. doi: 10.1016/j.jbiomech.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Austin N, Nilwik R, Herzog W. In vivo operational fascicle lengths of vastus lateralis during sub-maximal and maximal cycling. J Biomech. 2010;43:2394–2399. doi: 10.1016/j.jbiomech.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford OM, Jones DA. Measurement of fibre pennation using ultrasound in the human quadriceps in vivo. Eur J Appl Physiol Occup Physiol. 1992;65:433–437. doi: 10.1007/BF00243510. [DOI] [PubMed] [Google Scholar]
- 12.Lévénez M, Timmermans B, Duchateau J. Effect of myo-aponeurotic crocheting of the sural triceps on passive pressure and muscle architecture during stretching. Kinesither Rev. 2009;9:56–61. [Google Scholar]
- 13.Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. 2009;90:819–826. doi: 10.1016/j.apmr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Klimstra M, Dowling J, Durkin JL, MacDonald M. The effect of ultrasound probe orientation on muscle architecture measurement. J Electromyogr Kinesiol. 2007;17:504–514. doi: 10.1016/j.jelekin.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Kellis E, Galanis N, Kapetanos G, Natsis K. Architectural differences between the hamstring muscles. J Electromyogr Kinesiol. 2012;22:520–526. doi: 10.1016/j.jelekin.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Abellaneda S, Guissard N, Duchateau J. Relation entre les modifications de l’architecture musculo-tendineuse et le développement de la tension pendant l’étirement passif du triceps sural. Kinesither Rev. 2006;6:29–33. [Google Scholar]
- 17.Lévénez M, Timmermans B, Duchateau J. Effet du crochetage myo-aponévrotique du triceps sural sur la tension passive et l’architecture musculaire à l’étirement. Kinesither Rev. 2009;9:56–61. [Google Scholar]
- 18.Samukawa M, Hattori M, Sugama N, Takeda N. The effects of dynamic stretching on plantar flexor muscle-tendon tissue properties. Man Ther. 2011;16:618–622. doi: 10.1016/j.math.2011.07.003. [DOI] [PubMed] [Google Scholar]


