Fig. 9.
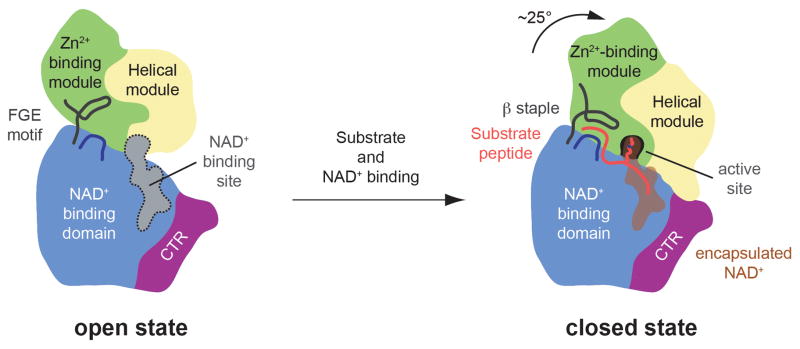
Model for the conformational changes and regulation of SIRT1. A cartoon representation of the apo SIRT1CAT•CTR heterodimer (left) and the SIRT1CAT•CTR•ADPR•Substrate complex (right), colored as in Fig. 1a. Substrate and co-factor binding leads to the closure of the SIRT1 catalytic domain. The substrate peptide is primarily bound by backbone interactions and the formation of a three-stranded anti-parallel β staple. The hydrophobic acetylated lysine substrate residue reaches into the secluded internal active site through the hydrophobic tunnel where it is oriented in close proximity to the activated NAD+. In the closed state, the CTR forms a salt-bridge with the helical module, thereby reducing the efficiency of catalysis.
