Abstract
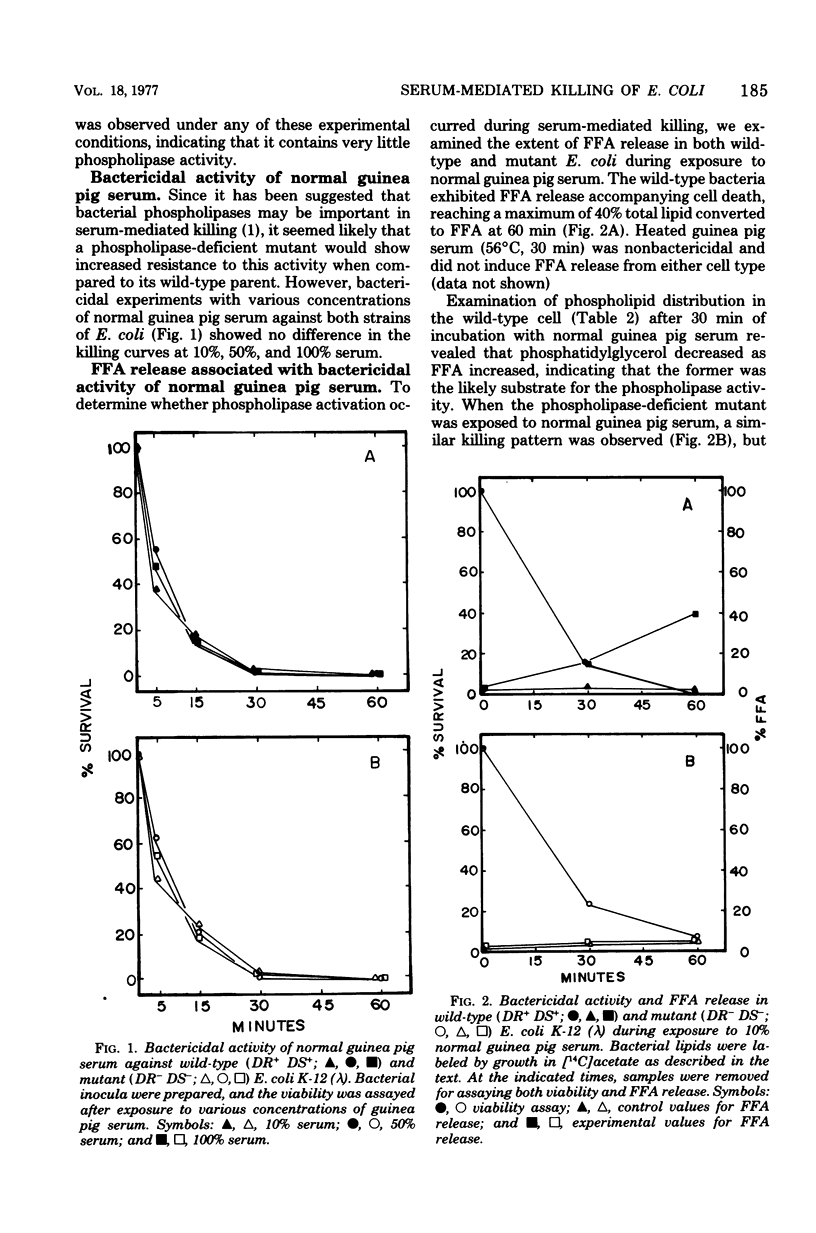
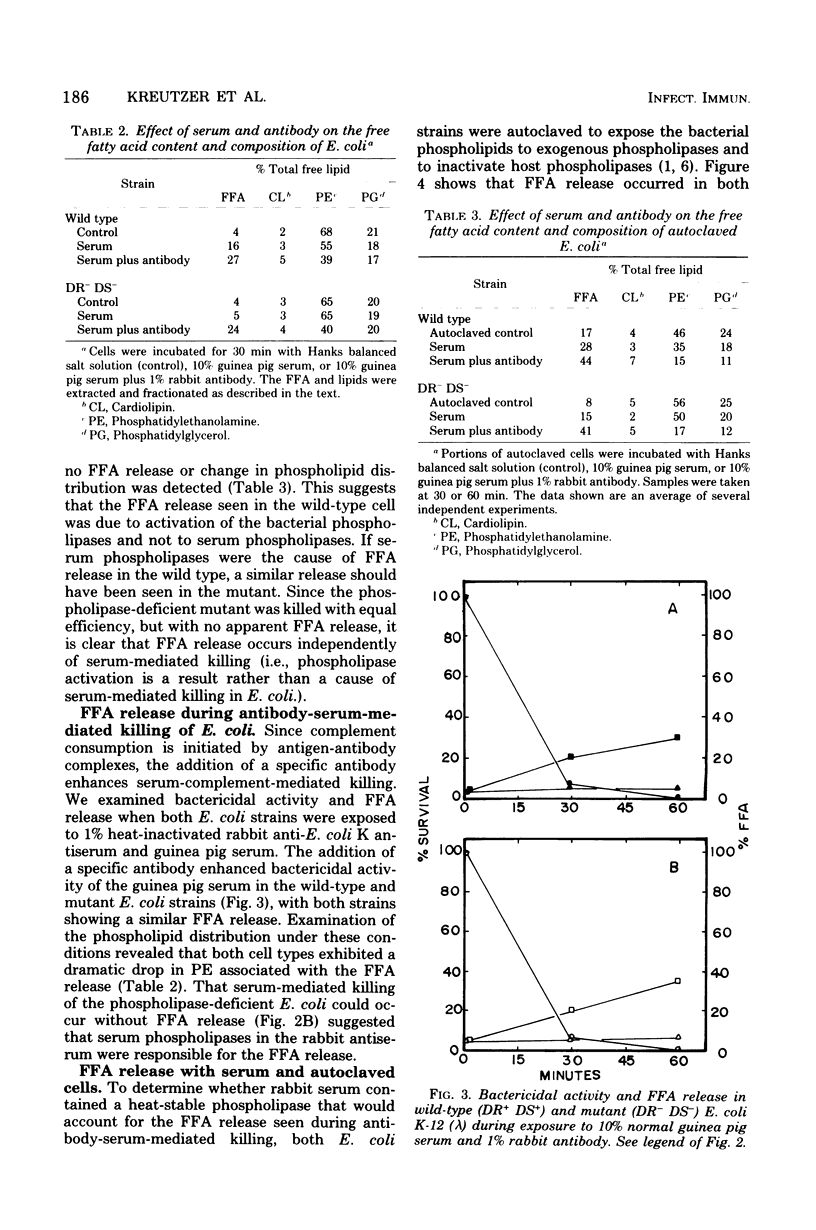
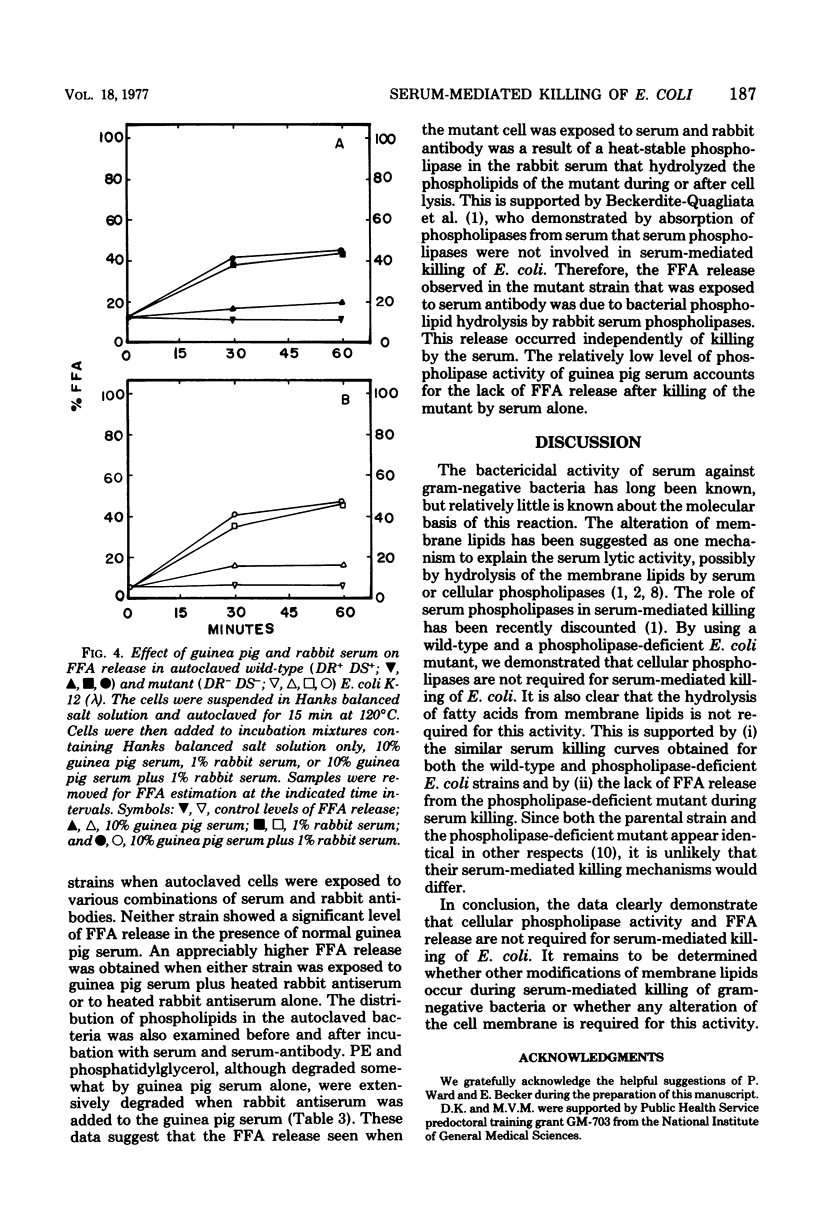
The importance of bacterial phospholipases during serum-mediated killing of Escherichia coli was examined by using wild-type DR+ DS+ and an isogenic phospholipase-deficient mutant DR- DS-. No difference in serum sensitivity was observed when the parental DR+ DS+ and mutant DR- DS- strains were exposed to various concentrations of normal guinea pig serum. Examination of the free fatty acid (FFA) and lipid composition during serum-mediated killing of the two E. coli strains indicated that FFA release occurred only in the parental DR+ DS+ strain. No FFA release or lipid degradation was detected in the mutant DR- DS- strain during serum killing. The addition of heat-inactivated E. coli antiserum (rabbit) to normal guinea pig serum caused FFA release in both E. coli strains. This FFA release was found to be independent of serum-mediated killing and due to a highly active and heat-resistant rabbit serum phospholipase that hydrolyzed the bacterial lipids after serum killing. The data presented indicate that serum-mediated killing of E. coli is independent of FFA release and that activation of bacterial phospholipases and the resulting release of FFA are only a result rather than a cause of serum-mediated cell death.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerdite-Quagliata S., Simberkoff M., Elsbach P. Effects of human and rabbit serum on viability, permeability, and envelope lipids of Serratia marcescens. Infect Immun. 1975 Apr;11(4):758–766. doi: 10.1128/iai.11.4.758-766.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Wulff D. L. A role for phospholipid hydrolysis in the lysis of Escherichia coli infected with bacteriophage T4. Virology. 1969 Jun;38(2):241–246. doi: 10.1016/0042-6822(69)90365-1. [DOI] [PubMed] [Google Scholar]
- Doi O., Oki M., Nojima S. Two kinds of phospholipase A and lysophospholipase in Escherichia coli. Biochim Biophys Acta. 1972 Feb 21;260(2):244–258. [PubMed] [Google Scholar]
- Duckworth D. H. The metabolism of T4 phage ghost-infected cells. I. Macromolecular synthesis and ransport of nucleic acid and protein precursors. Virology. 1970 Mar;40(3):673–684. doi: 10.1016/0042-6822(70)90212-6. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Pettis P. Phospholipase activity associated with serum albumin. Biochim Biophys Acta. 1973 Jan 19;296(1):89–93. doi: 10.1016/0005-2760(73)90047-7. [DOI] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Ahl L. A., Fisher M. W. Sensitivity of Pseudomonas aeruginosa to normal serum and to polymyxin. J Bacteriol. 1969 May;98(2):453–457. doi: 10.1128/jb.98.2.453-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. H., Buller C. S. Phospholipid metabolism in T4 bacteriophage infected Escherichia coli K-12 (lambda). J Virol. 1969 May;3(5):463–468. doi: 10.1128/jvi.3.5.463-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957 Dec;21(4):273–294. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F., Jr Lysis of Escherichia coli by ethylenediaminetetraacetate and phospholipases as measured by beta-galactosidase activity. J Bacteriol. 1967 Oct;94(4):934–941. doi: 10.1128/jb.94.4.934-941.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


