Abstract
The complex iridium oxide Na3Ir3O8 with a B-site ordered spinel structure was synthesized in single crystalline form, where the chiral hyper-kagome lattice of Ir ions, as observed in the spin-liquid candidate Na4Ir3O8, was identified. The average valence of Ir is 4.33+ and, therefore, Na3Ir3O8 can be viewed as a doped analogue of the hyper-kagome spin liquid with Ir4+. The transport measurements, combined with the electronic structure calculations, indicate that the ground state of Na3Ir3O8 is a low carrier density semi-metal. We argue that the semi-metallic state is produced by a competition of the molecular orbital splitting of t2g orbitals on Ir3 triangles with strong spin-orbit coupling inherent to heavy Ir ions.
The realization of quantum spin liquid is a long-sought dream in condensed matter physics, where exotic phenomena such as spinon Fermi surface, fractional excitations or unconventional superconductivity upon doping are anticipated1. The most promising arena for spin liquid is in geometrically frustrated lattices based on a triangular motif. Antiferromagnetically interacting spins on such a frustrated lattice cannot simultaneously satisfy all magnetic bonds and the interplay with quantum effect may give rise to a quantum liquid state of spins. To date experimental efforts have yielded several candidates, such as transition metal oxides with a kagome lattice1,2 and organic Mott insulators with a triangular lattice1,3.
A prime candidate in these recent discoveries is Na4Ir3O84. It is one of the very first candidates for a three-dimensional quantum spin liquid, where Ir4+ ions with a localized S = 1/2 moment (or most likely close to Jeff = 1/2 moment, see below) form a corner-sharing network of triangles in three-dimensions, called a “hyper-kagome” lattice. All the Ir sites and the Ir-Ir bonds are equivalent, rendering the hyper-kagome lattice magnetically frustrated. Indeed, Na4Ir3O8 exhibits no magnetic ordering down to 2 K despite the strong antiferromagnetic interaction inferred from the Curie-Weiss temperature θW ~ -650 K. This discovery triggered intensive experimental and theoretical surveys on this compound, including proposals for the presence of a spinon Fermi surface5.
In tandem with the discovery of Na4Ir3O8, complex Ir4+ oxides have recently been recognized as a novel playground for physics of strong spin-orbit coupling (SOC). SOC of Ir4+ is as large as λSO ~ 0.6 eV, reflecting the heavy atomic mass. λSO is significant even when compared with the other parameters dominating the electronic states, such as the inter-site hopping t, the Coulomb repulsion U and the crystal field splitting Δ. The interplay of the large SOC with the other parameters leads to the formation of unprecedented electronic phases. In the layered perovskite Sr2IrO4, for example, a half filled Jeff = 1/2 state is formed by strong SOC, which is a superposition of three t2g orbitals 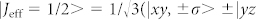 ,
, 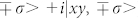 ) and gives rise to a spin-orbital Mott insulator6. The magnetic coupling of Jeff = 1/2 moments in such spin-orbital Mott insulators can be distinct from those of the spin-dominant moments in 3d oxides, as exemplified by the possible Kitaev spin-liquid proposed for honeycomb iridates7. Such unique magnetic couplings in iridates may make the mystery of the possible spin-liquid state of Na4Ir3O8 even more intriguing8.
) and gives rise to a spin-orbital Mott insulator6. The magnetic coupling of Jeff = 1/2 moments in such spin-orbital Mott insulators can be distinct from those of the spin-dominant moments in 3d oxides, as exemplified by the possible Kitaev spin-liquid proposed for honeycomb iridates7. Such unique magnetic couplings in iridates may make the mystery of the possible spin-liquid state of Na4Ir3O8 even more intriguing8.
The insulating state of Na4Ir3O8 is marginally stabilized by a modest U with the help of strong SOC. Such weak Mottness, implying the close proximity to a metallic state9, has been proposed to play a vital role in realizing the spin-liquid ground state here, as well as in organic triangular spin liquids1. It is natural to expect that Na4Ir3O8 might switch into a metallic state by carrier-doping or by applying pressure. Exotic superconductivity at the critical border to a spin liquid might be anticipated in analogy with the organic systems10. The metallic state of hyper-kagome iridate is therefore worthy of exploration.
Despite such intriguing outlooks in Na4Ir3O8, not much progress has been achieved in the critical investigation of the spin-liquid like state including the role of spin-orbit coupling, largely due to the lack of single crystals. During the course of attempting to grow single crystals of Na4Ir3O8, we obtained single crystals of Na3Ir3O8, a B-site ordered spinel. The crystal structure is distinct from that of Na4Ir3O8, a spin liquid candidate, but shares the same Ir-O hyper-kagome network. Na3Ir3O8 therefore can be viewed as a doped hyper-kagome spin liquid. We report here that Na3Ir3O8 has a semi-metallic ground state produced by a subtle competition of strong spin-orbit coupling and molecular orbital splitting of t2g electrons in an Ir3 triangular unit.
Results
Crystal Structure Analysis
Single crystals of Na3Ir3O8 were grown by a flux method (see Methods). The obtained crystals are stable in air. Detailed refinement of structure using single crystal X-ray diffraction was performed on the crystals. Satisfactory refinement was performed with space groups P4132 or P4332. The obtained structural parameters are listed in Table 1. The structure shown in Fig. 1a can be viewed as an ordered spinel, an intimately related but distinct structure to that of Na4Ir3O8. Rewriting the chemical formula of 1/2 Na3Ir3O8 as Na(Na1/4, Ir3/4)2O4, in correspondence with that of spinel AB2O4, is useful to understand the structure. Na2 in Table 1 corresponds to the tetrahedral A-site of spinel structure. In Na4Ir3O8, this site is empty and, instead, the octahedral A-site is occupied by Na1.5. The occupancies of the octahedral sites were found to be zero in the Na3Ir3O8 single crystal within the resolution of structural analysis. Na1 corresponds to 1/4 of the pyrochlore B sub-lattice. The remaining 3/4 of the pyrochlore B sub-lattice sites are occupied by Ir atoms. All the Ir sites are equivalent and form the same hyper-kagome network as in Na4Ir3O8. The presence of chirality in the hyper-kagome network gives rise to two possible space groups with different chiralities. The crystals under investigation contained racemic twins (see Supplementary). We did not find any signature of non-stoichiometry such as sodium deficiency in the refinement and therefore the composition of single crystal should be stoichiometric Na3Ir3O8.
Table 1. Refined structural parameters of Na3Ir3O8. The space group is P4132 (No. 213) and Z = 4, and the lattice constant is a = 8.9857(4) Å. g and Uiso denote site occupancy and isotropic displacement parameter, respectively. The final R indices are R = 0.0133 and wR = 0.0287.
| Atom | Site | g | x | y | z | Uiso (Å2) |
|---|---|---|---|---|---|---|
| Ir | 12d | 1 | 0.61264(1) | x + 1/4 | 5/8 | 0.00802(4) |
| Na1 | 4b | 1 | 7/8 | 7/8 | 7/8 | 0.0122(5) |
| Na2 | 8c | 1 | 0.2570(2) | x | x | 0.0138(4) |
| O1 | 8c | 1 | 0.1144(2) | x | x | 0.0105(6) |
| O2 | 24e | 1 | 0.1364(3) | 0.9071(2) | 0.9186(2) | 0.0111(4) |
Figure 1. Crystal structure of Na3Ir3O8.

(a) The unit cell of Na3Ir3O8 with a space group of P4132. The orange, yellow, silver and blue spheres represent Na1, Na2, Ir and O atoms, respectively19. While Na1 atoms are enclosed in distorted O6 octahedra, Na2 atoms are coordinated tetrahedrally by oxygen atoms. The iridium atoms comprise corner-sharing triangles in three-dimensions called hyper-kagome lattice. (b) Schematic illustration of the hyper-kagome lattice showing formation of molecular orbitals on Ir3 triangles seen in the scalar relativistic calculation. dzx on Ir(1) has hopping paths to Ir(2) and Ir(3) via O2 p-orbitals, forming a localized molecular orbitals. Likewise, dyz on Ir(1) forms another molecular orbital with the like orbitals on Ir(4) and Ir(5). Note that the t2g orbitals are defined in a local frame (sketched in the inset) which is unique for each Ir site. The hopping via O1, which is likely much smaller than the one via O217, is neglected here.
Transport and Magnetic Properties
The composition of Na3Ir3O8 corresponds to Ir valence of 4.33+, not 4+. Considering that Na4Ir3O8 is an Ir4+ (5d5) Mott insulator, Na3Ir3O8 may be viewed as 1/3 hole-doped hyper-kagome spin liquid. Indeed, the obtained single crystals were found to show metallic behavior of resistivity as shown in Fig. 2a, in marked contrast to the spin liquid Na4Ir3O8. The magnitude of resistivity is relatively large as a metal, ~1 mΩcm at 5 K. The Hall coefficient of Na3Ir3O8 indicates that the poorly metallic behavior originates from a low carrier concentration. The Hall coefficient shown in Fig. 2b is negative and its magnitude is orders of magnitude larger than those of typical metals, indicating that a very low density of electrons dominates the transport. The carrier number estimated from the Hall constant is of the order of 1019 cm−3 at 5 K, which is too small if the picture of a simple 1/3 hole-doped Mott insulator with 2/3 electrons is to be assumed. The Hall mobility at 5 K is as large as ~100 cm2/V·s and, clearly, the disorder is not the dominant factor of the poorly metallic behavior. The Hall coefficient shows a rapid decrease, more than one order of magnitude, with increasing temperature from 5 K to 300 K, despite the metallic behavior of resistivity. This very likely implies the coexistence of two different types of carriers at least at high temperatures, suggesting that Na3Ir3O8 is either a semi-metal or a very narrow gap semiconductor doped with a small number of carriers. The result of band calculation shown below indicates that the former is the case. Unexpectedly, the ground state of a doped hyper-kagome appears to be very close to a band insulator.
Figure 2. Transport, magnetic and thermodynamic properties of Na3Ir3O8.
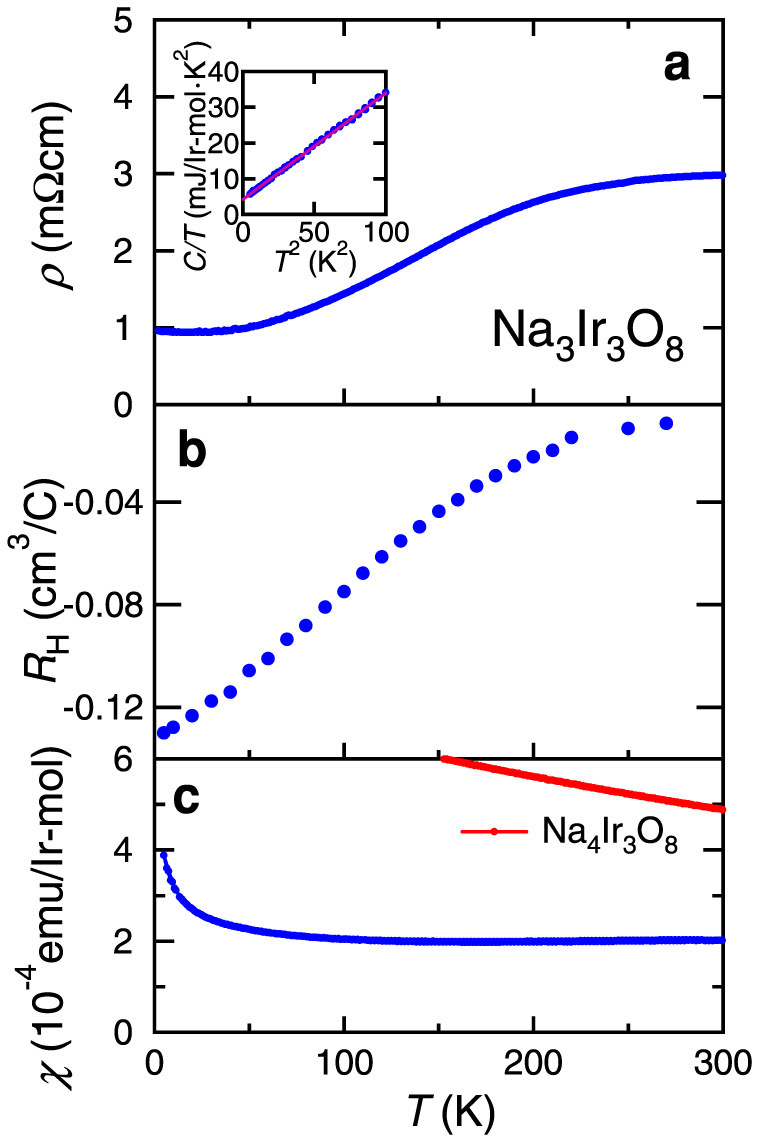
(a) Resistivity, (b) Hall coefficient and (c) Magnetic susceptibility of Na3Ir3O8 single crystal. The red dots in (c) represent magnetic susceptibility of Na4Ir3O8 polycrystalline sample for comparison. The inset in (a) shows specific heat C divided by temperature T of Na3Ir3O8. The solid line is a fit based on the conventional model C/T = γ + βT2.
The low temperature specific heat of Na3Ir3O8 single crystal, shown in the inset of Fig. 2a, yields an electronic specific heat coefficient γ = 4.3 mJ/Ir-mol·K2. This value is pronounced especially when the vanishingly small density of carriers is considered and therefore implies the presence of heavy mass carriers. The magnetic susceptibility (Fig. 2c) is almost temperature-independent, distinct from that of magnetic Na4Ir3O8. The paramagnetic susceptibility is estimated to be χPM = 2.7 × 10−4 emu/Ir-mol by subtracting the contribution of core diamagnetism taken from the values for Na+, Ir4+ and O2−. This value gives the Wilson ratio RW ~ 4.6 combined with γ = 4.3 mJ/Ir-mol·K2. The large RW at a glance would mean a Stoner enhancement and close proximity to ferromagnetism. Considering the small density of carriers, we speculate that the enhanced χPM might be ascribed alternatively to a superposition of Van Vleck-like contribution.
Resonant Inelastic X-ray Scattering
To verify the 1/3 hole-doped state, we performed a resonant inelastic X-ray scattering (RIXS) at L3-edge of Ir(2p3/2 → 5d)11 on Na3Ir3O8 single crystals and Na4Ir3O8 polycrystalline samples. In the obtained RIXS spectra shown in Fig. 3, three peaks at around 0.2, 1.0 and 4.0 eV are clearly observed. All those peaks show only small dispersions, which very likely originates from the intra-atomic excitations within the d-orbitals of Ir. The intra-atomic character of excitations justifies the comparison of spectra for the single crystal Na3Ir3O8 and the polycrystalline Na4Ir3O8. A clear shift of peaks at 1.0 eV and 4.0 eV to lower energy and a strong suppression of the 0.2 eV peak are clearly seen in Na4Ir3O8. The peak at 4.0 eV can be assigned to t2g → eg transitions, whereas the peaks at 1.0 eV and 0.2 eV likely originate from the excitations within the t2g manifold. The shift of 4.0 eV peak can be ascribed to the increased Ir-O distance (~3%) in Na4Ir3O8 and hence the reduced cubic splitting 10 Dq. The change in the 1.0 eV and 0.2 eV peaks cannot be accounted for simply by the crystal field effect and very likely reflects the difference of band filling between the two compounds. This point will be justified based on the result of band calculation described below, which supports the 1/3 hole-doped state in Na3Ir3O8.
Figure 3. RIXS spectra of Na3Ir3O8 single crystal and polycrystalline Na4Ir3O8 at Ir L3-edge.
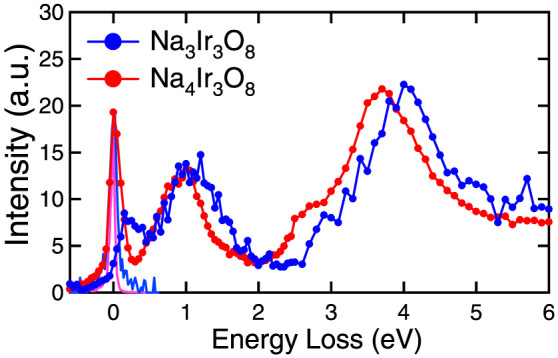
The two spectra were normalized by the intensity of the high energy tail above 6 eV. The blue and pink solid lines around zero energy show the normalized elastic signals independently measured with σ-polarized incident X-ray.
Electronic Structure Calculation
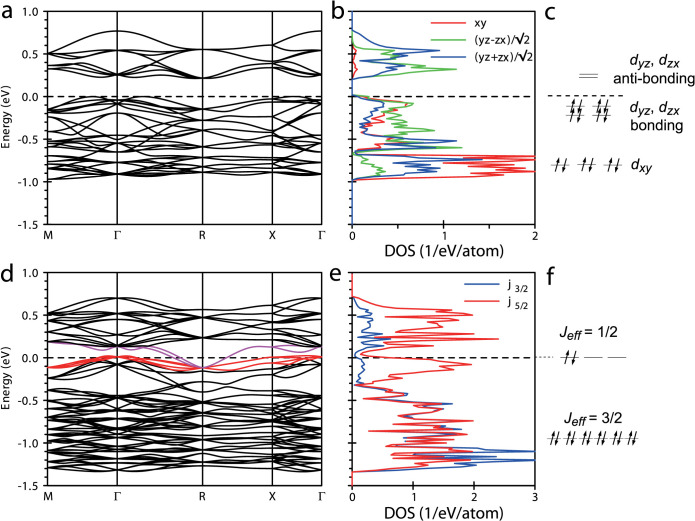
ab initio electronic structure calculation using a fully relativistic LMTO code12 revealed that Na3Ir3O8 is a compensated semi-metal due to the interplay of periodic potential and SOC, which is consistent with the experimental observation described above. Figure 4 depicts the electronic states around the Fermi energy where t2g orbitals of Ir have a dominant contribution. The 5d electrons are accommodated into the t2g manifold due to the large t2g – eg crystal field splitting. The number of d-electrons per Ir atom is non-integer, 4.67 (Ir4.33+). In the unit formula with 3 Ir atoms, we have even number of electrons, 14( = 3 × 4.67) t2g electrons. In the scalar-relativistic calculation neglecting SOC, shown in Fig. 4a, a well-defined gap of 0.2 eV can be seen within the t2g bands. 14 t2g electrons fill up all the bands below the 0.2 eV gap and the system is a band insulator. A similar gap separating t2g bands and explained by strong p-d hopping was also found for Na4Ir3O813, where, due to the higher Na content, the unoccupied t2g bands above the gap in Na3Ir3O8 are partially filled.
Figure 4. Electronic structure calculation for Na3Ir3O8.
(a) Scalar-relativistic band structure, and (b) orbital resolved Ir 5d density of states (DOS). The xy orbital represents a t2g orbital lying in the plane which contains two larger Ir-O1 bonds. (c) Schematic energy level splitting of the t2g orbitals in an Ir3 triangular molecule. The dxy orbitals have lower energy due to the crystal field. The dyz and dzx orbitals form bonding and anti-bonding molecular orbitals, which produce an energy gap. (d) Relativistic band structure including spin-orbit coupling (SOC). The bands which form hole and electron pockets are colored in red and magenta, respectively. (e) Ir 5d density of states corresponding to (d) resolved with j = 5/2 and 3/2 characters. (f) Local d-level splitting for an Ir3 triangle with SOC. At each Ir atom coordinated octahedrally by O atoms, SOC splits the t2g orbitals into lower lying Jeff = 3/2 quartet and upper Jeff = 1/2 doublet. 14 electrons in an Ir3 triangle fills up the Jeff = 3/2 states, while the Jeff = 1/2 states are occupied partially.
Strong SOC of Ir, in reality, splits the conduction and the valence bands substantially and a negative band gap is realized. The band structure calculated with SOC is shown in Fig. 4d. Since the crystal structure lacks inversion symmetry, SOC lifts the Kramers degeneracy everywhere except for time-reversal invariant points. In the presence of realistic SOC, a pair of unoccupied t2g bands, colored in magenta, bends down near the R point and becomes degenerate with the valence bands at the R point. This pronounced SOC effect results in the overlap of the pair of conduction band and the valence band colored in red, and creates two electron-like Fermi surfaces around the R point and four hole-like Fermi surfaces near the Γ point. Na3Ir3O8 is therefore a robust semi-metal, protected by the degeneracy at the R point. From the calculation, the masses of carriers are estimated; 1.4, 2.3, 3.7 and 5.6m0 for hole bands and 1.8m0 for two electron bands. The relatively heavy masses yield a large specific heat coefficient γcalc = 2.9 mJ/Ir-mol·K2, though the carrier density is small. The γcalc is close to and only 35% smaller than the experimentally observed γ = 4.3 mJ/Ir-mol·K2. The more dispersive electron bands with higher mobility should dominate the transport, again consistent with the negative sign of Hall constant experimentally observed. The recent observation of Fano resonances in the phonon spectra of Na3Ir3O8 provides further evidence for the semi-metallic state, where the electron-hole excitation continuum interferes with superimposed discrete phonon states14.
Discussion
The effect of SOC leads to the closure of the scalar relativistic gap in Na3Ir3O8. An inspection of orbitally resolved densities of Ir 5d states reveals that the gap closure is a consequence of the competition between SOC and the level splitting associated with the formation of molecular orbitals on the Ir3 triangle. We consider Ir3 triangular “molecules” which are the basic structural unit of the hyper-kagome network. The dominant hopping process between Ir t2g states in the hyper-kagome lattice is the one between the nearest neighbor Ir d-orbitals via oxygen p orbitals (tpdπ) which is likely much larger than the direct Ir d-d hopping15,16. There are two oxygen sites, O1 and O2, in Na3Ir3O8, forming the IrO6 octahedra, and the Ir-O bond lengths for those two sites are very different (Ir-O1: 2.053 Å, Ir-O2: 1.976 or 1.978 Å). Because of the contrasting Ir-O bond lengths, the hopping via O2 is expected to be significantly stronger than via O1 as in the case of Na4Ir3O817. Let us define for each Ir site its own local frame with the x and y axis lying in the plane of the IrO4 square with two more distant O1 ions (see inset of Fig. 1b). Among the three t2g orbitals of Ir, the dxy orbital should be energetically stabilized by the crystal field associated with the longer Ir-O1 distance. This can be clearly recognized in the orbitally resolved density of states shown in Fig. 4b. Since dxy bands are fully filled, only dyz and dzx orbitals are responsible for the bands right above and below the 0.2 eV gap.
Both dyz and dzx have the hopping path to t2g orbitals of the neighboring Ir atoms via O2 but it is spatially limited. dzx on Ir(1) can hop only to dzx on Ir(2) or Ir(3), whereas dyz only to dyz on Ir(4) or Ir(5), meaning that for each of the two orbitals the hopping paths via O2 are restricted to one of the two Ir3 triangles sharing common Ir(1). In the simplified picture which considers only the hopping via O2 2p orbitals, therefore, the dzxstates form molecular orbitals on the Ir(1)Ir(2)Ir(3) triangle, namely, two degenerate bonding and one anti-bonding molecular orbitals. The dyz on Ir(1) participates in molecular orbitals on the Ir(1)Ir(4)Ir(5) triangle. Note that dzx and dyz orbitals are nearly orthogonal to each other, which means that those molecular orbitals which belong to different triangles do not interact and are almost localized. Considering the energy levels of the Ir3 molecule with 3 × 3 = 9 t2g orbitals, 3 dxy orbitals have the lowest energy. The other two, dyz and dzx, orbitals form 4 bonding and 2 anti-bonding orbitals, as schematically shown in Fig. 4c. With 14 electrons per Ir3 triangle, 3 dxy and 4 bonding molecular orbitals are fully filled and the splitting of bonding and anti-bonding molecular orbitals gives rise to an energy gap.
SOC of heavy Ir 5d orbitals is very large ~0.6 eV and may reconstruct the electronic structure. In a cubic environment SOC splits three-fold degenerate t2g d-orbitals into 2:1 ratio of lower Jeff = 3/2 (admixture of j = 5/2 and 3/2 characters) and higher Jeff = 1/2 states (purely j = 5/2 character), which accommodate 12 and 6 electrons respectively for the Ir3 triangle, as shown in Fig. 4f. In the strong SOC limit, Jeff = 3/2 states are fully filled up and Jeff = 1/2 states are 1/3 filled. The splitting into Jeff = 3/2 and higher Jeff = 1/2 states can be seen in the relativistic calculation with SOC shown in Figs. 4d, e. The competition between the molecular orbital split and strong SOC should be responsible for suppression of the 0.2 eV gap in the scalar relativistic calculation. This is because SOC mixes all three t2g states, dzx and dyz forming two distinct molecular orbitals and dxy.
Based on the calculated electronic structure, we can reasonably assign the relevant d-d excitations to the peaks observed in the RIXS spectrum in Fig. 3. The peak structure observed around 1.0 eV corresponds to the excitations from the Jeff = 3/2 to the unoccupied Jeff = 1/2 bands. The peak around 0.2 eV originates from the transition between the occupied Jeff = 1/2 bands with remnant molecular bonding character and the unoccupied Jeff = 1/2 bands with remnant anti-bonding character, likely representing the strong inter-band transition between the pair of flat bands crossing EF. The absence of the 0.2 eV peak in the RIXS spectrum of Na4Ir3O8 is fully consistent with the filling of the unoccupied and flat Jeff = 1/2 bands associated with the removal of 1/3 hole and opening of Mott gap in Na4Ir3O8.
In summary, we successfully synthesized single crystals of hyper-kagome iridate Na3Ir3O8. Unlike the spin-orbital Mott insulator Na4Ir3O8, Na3Ir3O8 was found to be a semi-metal. The electronic structure calculation indicates that Na3Ir3O8 should be a band insulator with absence of SOC. The semi-metallic state is shown to arise from a competition between the splitting of molecular orbitals on Ir3 triangles and strong SOC. The semi-metallic Na3Ir3O8 might be an intriguing platform to test the possible non-trivial topological effects, associated with the frustrated and chiral geometry of the lattice and the negative gap produced by SOC. As an example, the breaking of inversion symmetry due to the chirality, together with strong SOC, produces a sizable Rashba splitting with a Dirac dispersion at the Γ point in the hole band, which harbors non-trivial Berry phase in electron transport18. We therefore believe that such material inheriting strong SOC and a unique lattice topology provides a tool to substantialize novel states of matter.
Method
Crystal growth and characterizations
Single crystals of Na3Ir3O8 were grown by a flux-growth technique. The mixture of Na2CO3, IrO2 and NaCl with a ratio of 5:1:20 was loaded in an alumina crucible, and heated up to 1075°C in an oxygen atmosphere. It was then slowly cooled to 1000°C with a cooling rate of 2°C/hour, and subsequently furnace-cooled down to room temperature. Black and block-shaped single crystals were found in the solidified melt. The polycrystalline samples of Na4Ir3O8 were synthesized by a conventional solid state reaction as a reference material. The crystal structure of single crystals was analyzed by X-ray diffraction using a three circle diffractometer (Bruker AXS) equipped with SMART APEX II CCD, and Mo Kα radiation (see Supplementary). Transport, magnetic and thermodynamic properties were measured with Quantum Design PPMS and MPMS.
In the obtained crystals, we in fact found a very small amount of insulating crystals besides the majority of metallic ones. The X-ray diffraction indicated that the insulating crystals also had the Na3Ir3O8 stoichiometry, and the spectroscopic measurements showed no difference between the metallic and insulating crystals. Judging from the poor quality of the insulating crystals, we suspect that the insulating crystals include domains of the Na4Ir3O8 phase and there might be a temperature window where Na4Ir3O8 phase is stabilized during the crystal growth. However, the growth of Na4Ir3O8 single crystal is a future perspective and out of the scope of this article.
Resonant inelastic X-ray scattering (RIXS)
Resonant inelastic X-ray scattering (RIXS) measurement was performed at BL11XU of SPring-8. Incident X-rays were monochromatized by a double-crystal Si(111) monochromator and a secondary Si(844) channel-cut monochromator. Horizontally scattered X-rays were analyzed by a diced and spherically-bent Si(844) crystal. The total energy resolution was 70 meV. The energy of the incident X-ray was tuned at 11.214 keV, which corresponds to the L3-edge of Ir (2p3/2 → t2g).
The samples used were single crystal Na3Ir3O8 and polycrystalline Na4Ir3O8. The spectra were collected at 20 K in Na3Ir3O8, whereas at 300 K in Na4Ir3O8. The difference in the measurement temperature should not change the overall spectra. The contribution of elastic scattering was evaluated by independently measuring the elastic signal with a σ-polarized configuration. It was almost negligible in Na3Ir3O8, whereas the strong quasi-elastic signal was seen around zero energy in the Na4Ir3O8 polycrystalline sample. This is possibly attributed to the elastic signal coming from concomitant Bragg reflections and/or to the phonon excitations inherent to the measurement at room temperature.
Author Contributions
T.T. and H.T. conceived and designed the project. T.T. and A.M. performed synthesis and magnetic and transport measurements. J.N. analyzed the crystal structure. K.I., M.Y., T.T., A.M. and J.M. measured and analyzed the RIXS data. A.Y. conducted electronic structure calculation. T.T., A.Y. and H.T. wrote the manuscript and all authors discussed and reviewed the paper.
Supplementary Material
Supplementary information
Dataset 1
Acknowledgments
We thank A. W. Rost and A. Kato for technical supports in the RIXS measurement and D. Casa for fabrication of Si(844) analyzer for RIXS. We are grateful to R. Perry, G. Khaliullin, G. Jackeli, A. Bangura, D. Pröpper, A. V. Boris and B. Keimer for fruitful discussion. This work was partly supported by Grant-in-Aid for Scientific Research (S) (Grand No. 24224010) and Grant-in-Aid for Young Scientists (B) (Grant No. 23740246). The synchrotron radiation experiments were performed at the BL11XU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2012B3502 and 2013A3502).
References
- Balents L. Spin liquids in frustrated magnets. Nature 464, 199–208 (2010). [DOI] [PubMed] [Google Scholar]
- Mendels P. & Bert F. Quantum kagome antiferromagnet ZnCu3(OH)6Cl2. Journal of the Physical Society of Japan 79, 011001 (2010). [Google Scholar]
- Shimizu Y., Miyagawa K., Kanoda K., Maesato M. & Saito G. Spin liquid state in an organic Mott insulator with a triangular lattice. Phys. Rev. Lett. 91, 107001 (2003). [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Nohara M., Aruga-Katori H. & Takagi H. Spin-liquid state in the S = 1/2 hyperkagome antiferromagnet Na4Ir3O8. Phys. Rev. Lett. 99, 137207 (2007). [DOI] [PubMed] [Google Scholar]
- Lawler M. J., Paramekanti A., Kim Y. B. & Balents L. Gapless spin liquids on the three-dimensional hyperkagome lattice of Na4Ir3O8. Phys. Rev. Lett. 101, 197202 (2008). [DOI] [PubMed] [Google Scholar]
- Kim B. J. et al. Phase-sensitive observation of a spin-orbital Mott state in Sr2IrO4. Science 323, 1329–1332 (2009). [DOI] [PubMed] [Google Scholar]
- Jackeli G. & Khaliullin G. Mott insulators in the strong spin-orbit coupling limit: from Heisenberg to a quantum compass and Kitaev models. Phys. Rev. Lett. 102, 017205 (2009). [DOI] [PubMed] [Google Scholar]
- Chen G. & Balents L. Spin-orbit effects in Na4Ir3O8: A hyper-kagome lattice antiferromagnet. Phys. Rev. B 78, 094403 (2008). [Google Scholar]
- Podolsky D., Paramekanti A., Kim Y. B. & Senthil T. Mott transition between a spin-liquid insulator and a metal in three dimensions. Phys. Rev. Lett. 102, 186401 (2009). [DOI] [PubMed] [Google Scholar]
- Kurosaki Y., Shimizu Y., Miyagawa K., Kanoda K. & Saito G. Mott transition from a spin liquid to a Fermi liquid in the spin-frustrated organic conductor κ-(ET)2Cu2(CN)3. Phys. Rev. Lett. 95, 177001 (2005). [DOI] [PubMed] [Google Scholar]
- Ishii K. et al. Momentum-resolved electronic excitations in the Mott insulator Sr2IrO4 studied by resonant inelastic X-ray scattering. Phys. Rev. B 83, 115121 (2011). [Google Scholar]
- Antonov V., Harmon B. & Yaresko A. Electronic Structure and Magneto-Optical Properties of Solids (Kluwer Academic Publishers, Dordrecht, Boston, London, 2004). [Google Scholar]
- Norman M. R. & Micklitz T. Electronic structure of hyper-kagome Na4Ir3O8. Phys. Rev. B 81, 024428 (2010). [Google Scholar]
- Pröpper D. et al. Fano resonance in the infrared spectra of phonons in hyper-kagome Na3Ir3O8. Phys. Rev. Lett. 112, 087401 (2014). [Google Scholar]
- Shitade A. et al. Quantum spin Hall effect in a transition metal oxide Na2IrO3. Phys. Rev. Lett. 102, 256403 (2009). [DOI] [PubMed] [Google Scholar]
- Mazin I. I., Jeschke H. O., Foyetsove K., Valenti R. & Khomskii D. I. Na2IrO3 as a molecular orbital crystal. Phys. Rev. Lett. 109, 197201 (2012). [DOI] [PubMed] [Google Scholar]
- Micklitz T. & Norman M. R. Spin Hamiltonian of hyper-kagome Na4Ir3O8. Phys. Rev. B 81, 174417 (2010). [Google Scholar]
- Murakawa H. et al. Detection of Berry's phase in a bulk Rashba semiconductor. Science 342, 1490–1493 (2013). [DOI] [PubMed] [Google Scholar]
- Momma K. & Izumi F. Vesta 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 44, 1272–1276 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Dataset 1