Figure 4.
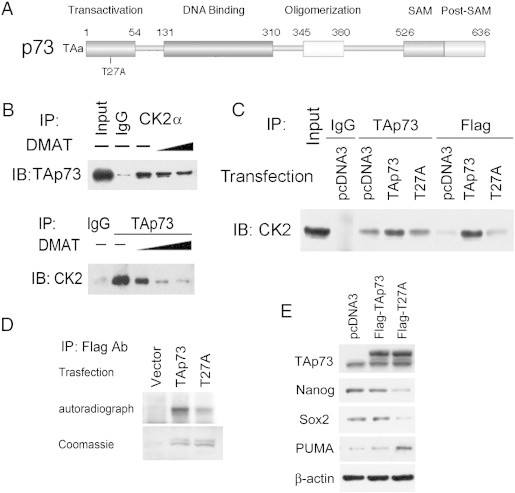
CK2 and TAp73 interaction is inhibited by CK2 inhibitor DMAT or T27A point mutation within a predicted CK2 phospho-acceptor motif in TAp73. A. CK2 interaction with TAp73 is predicted by presence of a high probability CK2 phosphorylation site at Threonine 27 (T27) within the TA domain of human TAp73 using Motifscan (Suppl Figure 4). B. Immunoprecipitation (IP) with anti-CK2α or TAp73 antibodies demonstrates reciprocal interaction between CK2α and TAp73 on immunoblotting (IB) in whole cell lysates of UM-SCC-46 cells. The interaction is decreased after treatment with increasing amount of CK2 specific inhibitor DMAT (10, 20 μM). C. Interaction between CK2α and TAp73 is decreased 48 hours after transfection with Flag-T27A-TAp73 mutant when compared with Flag-TAp73 control. Whole cell lysates from UM-SCC-46 cells were immunoprecipitated (IP) with TAp73 or Flag antibodies, and then immunoblotted (IB) with CK2α antibody. Physical interaction between CK2α and TAp73 was increased after over-expression of wild type TAp73, but decreased between Flag-T27A and CK2α. D. In vitro kinase assay shows decreased phosphorylation of TAp73 after T27A mutation. Lysates from cells transfected with empty vector, Flag-TAp73, or Flag-T27A were incubated with the recombinant CK2α2β2 protein in the presence of [γ-32P]ATP. The reaction mixtures were separated by SDS-PAGE and subjected to autoradiography (top panel). Bottom panel shows the Coomassie Brilliant Blue staining of the Flag-TAp73 fusion proteins as the loading control. E. Top panel, equivalent expression of Flag-TAp73 and Flag-T27A TAp73 in lysates used for C, D, E, obtained 48 hours after UM-SCC-46 cells were transfected with empty vector, wild type TAp73, or T27A mutant form of TAp73; lower panels, T27A TAp73 enhances inhibition of CSC markers and expression of proapoptotic protein PUMA. The protein expression of TAp73, Nanog, Sox2 and PUMA in whole cell lysates were assessed by Western blot, with β-actin as the loading control.
