Figure 3.
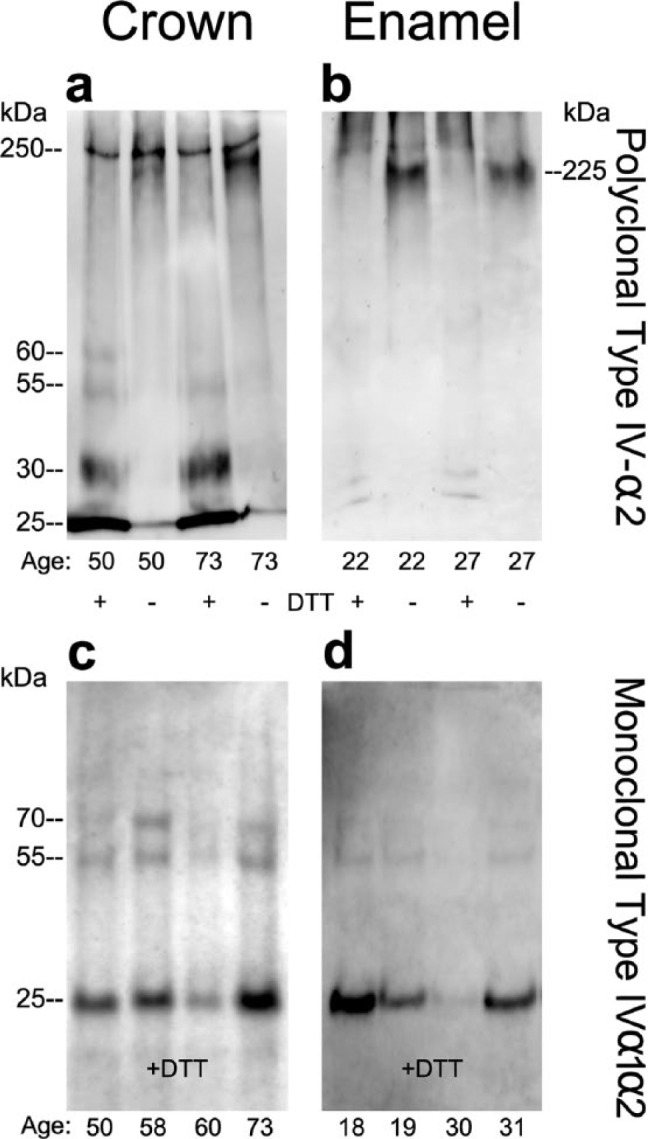
Immunoblotting of whole-crown and enamel organic matrix extracts identified intact type IV collagen α2-chains and associated fragments. Whole-crown extracts and enamel extracts (see ‘Methods’), from donors of different ages, were subjected to electrophoresis under either reducing or non-reducing conditions with 7.5% gels (a, b) or 4% to 20% linear gradient gels (c, d) and electroblotted onto polyvinylidene difluoride membranes. Blots were then probed with polyclonal (a-b) or monoclonal (c-d) anti-type IV collagen antibodies. (a-b) Under non-reducing conditions, intact type IV collagen α2-chains migrated as characteristic monomeric bands at 250 and 225 kDa. After reduction, smaller (30-25 kDa) fragments were detected more prominently in the crown extracts compared with enamel organic matrix extracts. (c-d) Both whole-crown extracts and enamel organic matrix extracts contained fragments of 25 kDa under reducing conditions when probed with monospecific anti-type IV collagen antibodies. Protein amounts loaded onto gel lanes for whole-crown and enamel organic matrix extracts were 15 μg and 30 μg, respectively. Antibodies used: polyclonal anti-type IV collagen antibody specific to the α2(IV) chain and monoclonal anti-type IV collagen antibody, which recognizes the helical domain of α1(IV) and α2(IV) chains. Molecular-weight estimates were based on globular standards co-electrophoresed on the same gel.
