Abstract
Background
Hepatic venous pressure gradient (HVPG) is a prognostic marker in cirrhosis, but is invasive. There is a need to validate a noninvasive marker to measure portal hypertension. Aspartate aminotransferase/platelet ratio index (APRI) is proposed as a good noninvasive estimator of hepatic fibrosis. Whether APRI could be used as noninvasive tool to measure portal hypertension has not been studied.
Aim
To correlate APRI with HVPG in patients with cirrhosis and to determine the diagnostic usefulness of the APRI in detection of high portal pressure.
Methods
APRI and HVPG were measured in consecutive patients of cirrhosis aged 18–75 years, with serum bilirubin <5 mg/dl, Child–Turcotte–Pugh (CTP) score ≤12, and without evidence of acute-on-chronic liver failure or flare.
Results
This study included 74 patients (median age 47 years, range 20–70 years; 57 males, (77%). The aetiology of cirrhosis was: viral 33 (45%), alcohol 10 (14%), and cryptogenic and others 31 (42%). The median HVPG was 16 mmHg (range 2–28 mmHg). The median APRI was 1.19 (range 0.17–7.92). There was significant correlation between HVPG and APRI (Spearman’s rho 0.365; p = 0.001). The ROC curve to study the performance of APRI for predicting high portal pressure (HVPG >12 mmHg) had area under curve 0.716 (95% CI 0.574–0.858). An APRI of ≥1.09 had a sensitivity 66%, specificity 73%, positive predictive value 85%, negative predictive value 47%, and diagnostic accuracy 68% for predicting HVPG >12 mmHg.
Conclusions
APRI correlates fairly with HVPG in patients of cirrhosis. An APRI score of ≥1.09 seems to have an acceptable accuracy for prediction of high portal pressure. APRI is a fair, bedside, cost-effective parameter for diagnosis of high portal pressure in patients with cirrhosis.
Keywords: Cirrhosis, HVPG, platelets, noninvasive, portal hypertension, portal pressure
Introduction
The development of portal hypertension is a common consequence of chronic liver diseases leading to the formation of oesophageal and gastric varices responsible for variceal bleeding, and it is associated with a high mortality rate as well as other severe complications such as portosystemic encephalopathy and sepsis.1 Measurement of hepatic venous pressure gradient (HVPG) is currently the best available method and is considered the gold standard for portal hypertension assessment in patients with cirrhosis.1,2 HVPG is a prognostic marker in patients with cirrhosis and complications of cirrhosis generally parallel with an increase in HVPG.
However, HVPG measurement is invasive and is routinely available and/or performed with adequate standards only in expert centres.1 There is thus a need for noninvasive methods able to predict, with acceptable diagnostic accuracy, the progression of portal hypertension towards high portal pressure (HVPG ≥2 mmHg). The aspartate aminotransferase/platelet ratio index (APRI) is proposed as a good noninvasive estimator of hepatic fibrosis. APRI was introduced in 2003 by Wai et al. as a simple noninvasive index which can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C.3 Subsequently APRI was validated as a noninvasive tool for diagnosis of significant fibrosis and cirrhosis of various other aetiologies.4–8
Whether APRI can be used as a noninvasive tool to measure portal hypertension has not been studied. In this prospective study, we aimed to correlate the APRI with HVPG in patients with cirrhosis with portal hypertension and to determine the diagnostic usefulness of the APRI in the detection of high portal pressure.
Patients and methods
Patients
The study was conducted in the Gastroenterology Department of GB Pant Hospital, New Delhi, India. Consecutive patients admitted with signs, symptoms, or investigations suggestive of cirrhosis of liver, aged 18–75 years, and with serum bilirubin <5 mg/dl, Child–Turcotte–Pugh (CTP) score ≤12, and without evidence of acute-on-chronic liver failure9 were prospectively enrolled in the present study. The exclusion criteria were as follows: (i) hepatic encephalopathy; (ii) spontaneous bacterial peritonitis; (iii) renal failure with serum creatinine >2 mg/dl; (iv) aspartate aminotransferase (AST) >5 × upper limit of normal (ULN); (v) any malignancy; (vi) history of surgery for portal hypertension or transjugular intrahepatic portosystemic shunt; (vii) underlying severe cardiac, respiratory, or psychiatric illness; (viii) concomitant treatment with beta-blockers, nitrates, or any other pharmacotherapy for prevention of variceal bleed; and (ix) refusal to participate in the study.
A written informed consent was obtained from all the included patients. The institutional ethics committee approved the study protocol.
Evaluation
Each included patient was evaluated by a detailed clinical history and a thorough physical examination. An attempt was made to determine the aetiology of cirrhosis. Oesophageal varices were classified at endoscopy as small (<3 mm) or large (≥3 mm). A history of variceal bleeding was sought. Complete blood count, liver function tests, kidney function tests, and ultrasound abdomen evaluation were done and severity of cirrhosis was assessed by CTP score. APRI estimation and HVPG measurement were done for each patient.
Aspartate aminotransferase/platelet ratio index
To calculate APRI, serum AST and platelet count were used, both of which were obtained simultaneously at the time of admission and prior to HVPG. APRI was calculated according to the formula proposed by Wai et al. in 2003:3 (AST of the sample/ULN of AST) × 100/platelets. The ULN for AST in our laboratory is 40 IU.
Hepatic venous pressure gradient
HVPG measurement was done after overnight fast, and under antibiotic cover. If the patient was on pharmacological treatment for portal hypertension (e.g. beta-blockers) it was temporarily withheld for 7 days prior to HVPG measurement. Under local anaesthesia, a 7 F central venous catheter (Arrow Medical, Athens, TX, USA) was placed in the right femoral vein or internal jugular vein under fluoroscopic guidance using the Seldinger technique. HVPG was measured by the standard technique10 in which a balloon catheter was introduced into the right hepatic vein under fluoroscopic guidance. The zero reference point was set at the mid-axillary point. The free hepatic venous pressure (FHVP) was obtained by keeping the catheter free into the lumen of the hepatic vein. The balloon of the catheter was then inflated to wedge the lumen of hepatic vein. Presence of wedging was confirmed by absence of reflux into the inferior vena cava, after the injection of 2 ml intravenous contrast, and appearance of a sinusoidogram. The pressure tracing at this juncture showed absence of wave forms and the pressure was labelled as wedged hepatic venous pressure (WHVP). HVPG was determined by subtracting free from wedged hepatic venous pressures (WHVP – FHVP). All measurements were performed in duplicate. If the difference between the two readings was more than 1 mmHg, all the readings were discarded and fresh set of measurements were taken. The normal accepted value for HVPG in most experienced haemodynamic laboratories is within 1–5 mmHg.
Statistical methods
Data in the study was reported as median (range) or n (%). This study was designed to correlate HVPG values with APRI score. Bivariate Spearman’s rank correlation coefficients were used to assess the association between the APRI and HVPG. A receiver operating characteristics (ROC) curve was plotted to study the performance of APRI in predicting high portal pressure (HVPG >12 mmHg). The best cut-off value of APRI to predict high portal pressure was obtained by calculating the Youden’s index. Based on the cut-off obtained the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of APRI in predicting high portal pressure was calculated. Statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL, USA).
Results
Patients
From July 2008 to January 2010, 222 consecutive patients with signs, symptoms, or investigations suggestive of cirrhosis of liver, aged between 18–75 years, with serum bilirubin <5 mg/dl, CTP score ≤12, and without evidence of acute-on-chronic liver failure were prospectively enrolled in the study. There were 148 patients who were excluded due to one or more of the following reasons: (i) hepatic encephalopathy (n = 34); (ii) spontaneous bacterial peritonitis (n = 16); (iii) renal failure with serum creatinine >2 mg/dl (n = 27); (iv) AST >5 × ULN (n = 1); (v) any malignancy (n = 12); (vi) history of surgery for portal hypertension or transjugular intrahepatic portosystemic shunt (n = 5); (vii) underlying severe cardiac, respiratory, or psychiatric illness (n = 6); (viii) concomitant treatment with beta-blockers, nitrates, or any other pharmacotherapy for prevention of variceal bleed (n = 29); and (ix) refusal to participate in the study (n = 18). Hence, the remaining 74 patients were included in the study.
Patient characteristics
The patient characteristics are shown in Table 1. The median age of the included patients was 47 (range 20–70) years and 57 patients (77%) were males. The aetiology of cirrhosis was viral in 33 (45%) patients, alcohol in 10 (14%) patients, and cryptogenic or others in 31 (42%) patients. Most (91%) patients had oesophageal varices but history of variceal bleeding was present in only 24% patients. The median CTP score was 8 (range 5–12). Ascites was present in 21 (28%) patients. The median HVPG was 16 (range 2–28) mmHg. The median APRI was 1.19 (range 0.17–7.92).
Table 1.
Patient characteristics
| Characteristic | Study population (n = 74) |
|---|---|
| Age (years) | 47 (20–70) |
| Sex | |
| Male | 57 (77) |
| Female | 17 (23) |
| Aetiology of cirrhosis | |
| Viral | 33 (45) |
| Alcohol | 10 (14) |
| Cryptogenic or others | 31 (42) |
| Oesophageal varices | |
| Large | 38 (51) |
| Small | 30 (40) |
| None | 6 (8) |
| History of variceal bleeding | 18 (24) |
| Body mass index (kg/m2) | 21.5 (13.9–37.5) |
| Ascites | 21 (28) |
| CTP score | 8 (5–12) |
| Haemoglobin (g/dl) | 10.8 (6.9–16.8) |
| White blood count ( × 109/l) | 4.9 (1.7–10.3) |
| Platelet count (×109/l) | 133 (36–474) |
| ALT (IU/l) | 46 (11–250) |
| AST (IU/l) | 60 (20–177) |
| Serum albumin (g/dl) | 3.3 (2.1–4.8) |
| Serum bilirubin (mg/dl) | 1.4 (0.4–4.6) |
| Prothrombin time prolongation (s) | 5 (0–29) |
| Serum creatinine (mg/dl) | 0.8 (0.3–1.4) |
| Serum sodium (meq/l) | 136 (120–145) |
| HVPG (mmHg) | 16 (2–28) |
| APRI | 1.19 (0.17–7.92) |
Values are median (range) or n (%).
ALT, alanine aminotransferase; APRI, aspartate aminotransferase/platelet ratio index; AST, aspartate aminotransferase; CTP, Child–Turcotte–Pugh; HVPG, hepatic venous pressure gradient
Correlation of APRI with HVPG
There was significant correlation between HVPG and APRI (Spearman’s rho 0.365; p = 0.001). Figure 1 shows the scatter plot of HVPG and APRI. There was a significant difference in median APRI between patients with HVPG ≤12 mmHg and those with HVPG >12 mmHg: 0.70 (range 0.17–7.92) vs. 1.38 (range 0.34–6.49); p = 0.004, respectively (Figure 2).
Figure 1.
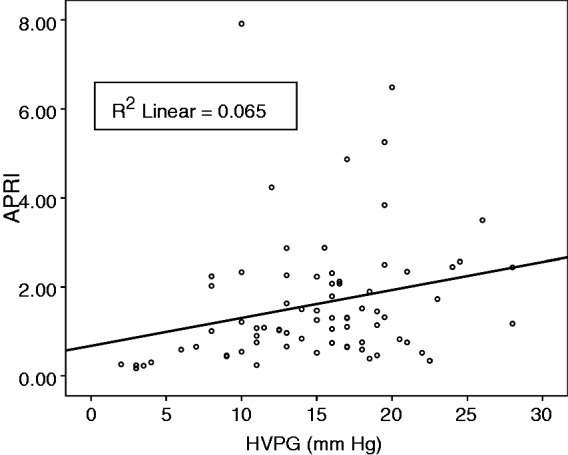
Scatterplot of hepatic venous pressure gradient (HVPG) vs. aspartate aminotransferase/platelet ratio index (APRI).
Figure 2.
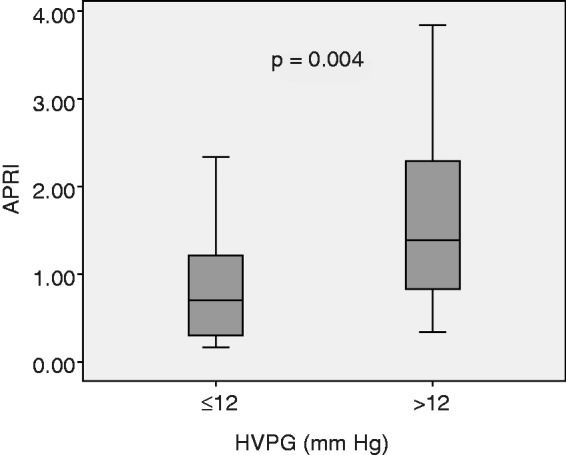
Boxplot showing median aspartate aminotransferase/platelet ratio index (APRI) scores of patients with hepatic venous pressure gradient (HVPG) ≤12 and >12 mmHg.
Prediction of high portal pressure (HVPG >12 mmHg)
When the ROC curve was plotted to study the performance of APRI for predicting high portal pressure (HVPG >12 mmHg), the area under curve was 0.716 (95% CI 0.574–0.858; Figure 3). The maximum Youden’s index was 0.387 which corresponded to a cut-off value of 1.09 of APRI. An APRI of ≥1.09 had a sensitivity 66%, specificity 73%, positive predictive value 85%, negative predictive value 47%, and diagnostic accuracy 68% for predicting HVPG >12 mmHg.
Figure 3.
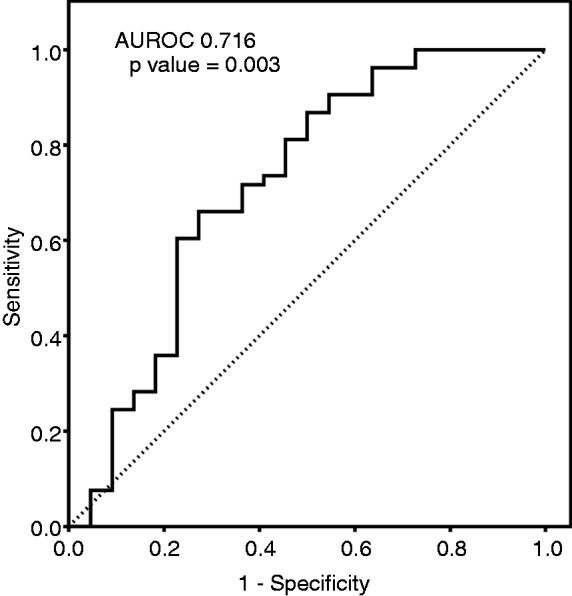
ROC curve of aspartate aminotransferase/platelet ratio index for predicting hepatic venous pressure gradient >12 mmHg.
Discussion
Our study has showed that APRI correlates well with HVPG in patients of cirrhosis. The APRI scores in patients with high portal pressure (HVPG >12 mmHg) were significantly higher than APRI scores in patients with lower HVPG. An APRI score of ≥1.09 seems to have an acceptable accuracy for prediction of high portal pressure and can be used as surrogate marker in settings where HVPG measurement is not available.
APRI was proposed as a simple and noninvasive predictor of liver fibrosis. It has several advantages. First, it is readily available because AST and platelets counts are part of the routine tests in managing patients with chronic liver disease. No additional blood tests or cost is needed. Second, it is easy to compute, without the use of complicated formula. Third, and more importantly, it is backed by sound pathogenesis. A more advanced state of fibrosis is associated with lower level of platelets through lower production of thrombopoietin, as well as higher portal hypertension and enhanced pooling and sequestration of platelets in the spleen.3,11
The development of portal hypertension is a common consequence of chronic liver diseases leading to the formation of oesophageal and gastric varices and is associated with a high mortality rate as well as other severe complications such as portosystemic encephalopathy and sepsis.1 Measurement of HVPG is currently the best available method and is considered the gold standard for portal hypertension assessment in patients with cirrhosis.1,2 Clinically significant portal hypertension is defined as an increase in HVPG to ≥10 mmHg; above this threshold, complications of portal hypertension might begin to appear. Measurement of HVPG is increasingly used in clinical hepatology, and numerous studies have demonstrated that the parameter is a robust surrogate marker for hard clinical end points.2 The main clinical applications for HVPG include diagnosis, risk stratification, identification of patients with hepatocellular carcinoma who are candidates for liver resection, monitoring of the efficacy of medical treatment, and assessment of progression of portal hypertension.2 Portal pressure measurement by means of HVPG allows stratifying cirrhosis in stages with defined outcomes, prognosis, and management strategies.12
However, HVPG measurement is invasive and is routinely available and/or performed with adequate standards only in expert centres.1 There is thus a need for noninvasive methods able to predict, with acceptable diagnostic accuracy, the progression of portal hypertension towards the levels of clinically significant (HVPG ≥10 mmHg) and severe (HVPG ≥12 mmHg) as well as the presence and the size of ooesophageal varices. Recently, transient elastography has been promoted as a novel noninvasive technology that allows measuring liver stiffness and that has gained popularity over the past few years. Although transient elastography has been initially proposed to assess liver fibrosis, a good correlation has been reported between liver stiffness values and HVPG as well as the presence of ooesophageal varices, suggesting that it could be an interesting tool for the noninvasive evaluation of portal hypertension. But like HVPG, transient elastography is also available in only tertiary care centres and is expensive. Hence, there is an urgent need to assess a simple, noninvasive parameter, which is widely available and can be used as a surrogate marker for HVPG. To this affect, APRI seems to be a fair option.
To our knowledge, this is the first study which has correlated HVPG with APRI. In 74 patients of cirrhosis of varied aetiology, APRI had a fair correlation with HVPG. And it could also be used with a fair accuracy in diagnosing patients with high portal pressure (HVPG >12 mmHg). A APRI of ≥1.09 had a sensitivity 66%, specificity 73%, positive predictive value 85%, negative predictive value 47%, and diagnostic accuracy 68% for predicting HVPG >12 mmHg. In a healthy individual, the highest level of APRI could be 0.67 taking AST as the ULN and platelets as the lowest limit of normal (150 × 109/l). A value of more than 0.67 signifies development of fibrosis. The original study by Wai et al.3 suggested that, for prediction of cirrhosis, an APRI value of >1 has a sensitivity of 89%. With the onset of cirrhosis, HVPG usually rises above normal (>5 mmHg). The suggestion by our study that high portal pressure can be expected at an APRI level ≥1.09, correlates well with the study by Wai et al.3
The progression of liver disease leads to decreased platelet count and increased AST level and has been reported in many studies. With worsening portal hypertension, there is increased sequestration and destruction of platelets in the enlarging spleen.13 Also, with progression of liver fibrosis there is decreased production of thrombopoietin by hepatocytes and, hence, reduced platelet production.14,15 Progression of liver fibrosis reduces the clearance of AST, leading to increased serum AST levels.16 In addition, advanced liver disease may be associated with mitochondrial injury, resulting in more marked release of AST, which is present in mitochondria and cytoplasm, relative to alanine aminotransferase.3,17,18
One important limitation of APRI is that any condition which raises the value of AST other than chronic hepatitis and cirrhosis would give a falsely higher value of APRI. Such condition could be any acute hepatitis or acute-on-chronic liver failure. In our study we excluded such patients by excluding patients with serum bilirubin ≥5 mg/dl and AST >5 × ULN. Another caution to exercise while using APRI is that APRI is not meant to replace the assessment of portal hypertension by use of standard tests such as HVPG or upper gastrointestinal endoscopy. Its best use is as a rough, bedside prediction of significant portal hypertension and prioritizing those patients for further investigations. Since a APRI value ≥1.09 has a PPV of 85%, most of these patients are expected to have high portal pressure and can be urgently subjected to further investigations and put on portal-pressure-reducing drugs. The third important limitation of our study is the small sample size with mixed aetiologies. Since aetiology of cirrhosis may have a significant impact on APRI values, it is imperative that further studies should be conducted to derive specific cut offs for individual aetiologies such as alcohol, viral, and cryptogenic.
In conclusion, our study has showed a fair correlation between APRI and HVPG in cirrhosis patients. A APRI score ≥1.09 seems to have an acceptable accuracy for prediction of high portal pressure. APRI can be a fair, bedside, cost-effective parameter for diagnosis of high portal pressure in patients with cirrhosis. Further large studies should be performed to test the correlation of APRI with complications of portal hypertension, such as oesophageal varices and variceal bleeding.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
Presentation
The abstract of this study was presented at the DDW 2011, Chicago, USA.
References
- 1.Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 2012; 56: 696–703 [DOI] [PubMed] [Google Scholar]
- 2.Bosch J, Abraldes JG, Berzigotti A, et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 2009; 6: 573–582 [DOI] [PubMed] [Google Scholar]
- 3.Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518–526 [DOI] [PubMed] [Google Scholar]
- 4.Shin WG, Park SH, Jun S-Y, et al. Simple tests to predict hepatic fibrosis in nonalcoholic chronic liver diseases. Gut Liver 2007; 1: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin WG, Park SH, Jang MK, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis 2008; 40: 267–274 [DOI] [PubMed] [Google Scholar]
- 6.Forestier J, Dumortier J, Guillaud O, et al. Noninvasive diagnosis and prognosis of liver cirrhosis: a comparison of biological scores, elastometry, and metabolic liver function tests. Eur J Gastroenterol Hepatol 2010; 22: 532–540 [DOI] [PubMed] [Google Scholar]
- 7.Kruger FC, Daniels CR, Kidd M, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J 2011; 101: 477–480 [PubMed] [Google Scholar]
- 8.Yilmaz Y, Yonal O, Kurt R, et al. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease: APRI in chronic liver disease. Hepat Mon 2011; 11: 103–106 [PMC free article] [PubMed] [Google Scholar]
- 9.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009; 3: 269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 2004; 39: 280–282 [DOI] [PubMed] [Google Scholar]
- 11.Shiha G, Sarin SK, Ibrahim AE, et al. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009; 3: 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albilllos A, Garcia-Tsao G. Classification of cirrhosis: the clinical use of HVPG measurements. Dis Markers 2011; 31: 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of ‘hypersplenic’ thrombocytopenia. J Clin Invest 1966; 45: 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki T, Takeshita A, Souda K, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol 1999; 94: 1918–1922 [DOI] [PubMed] [Google Scholar]
- 15.Adinolfi LE, Giordano MG, Andreana A, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol 2001; 113: 590–595 [DOI] [PubMed] [Google Scholar]
- 16.Kamimoto Y, Horiuchi S, Tanase S, et al. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology 1985; 5: 367–375 [DOI] [PubMed] [Google Scholar]
- 17.Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002; 122: 366–375 [DOI] [PubMed] [Google Scholar]
- 18.Nalpas B, Vassault A, Le Guillou A, et al. Serum activity of mitochondrial aspartate aminotransferase: a sensitive marker of alcoholism with or without alcoholic hepatitis. Hepatology 1984; 4: 893–896 [DOI] [PubMed] [Google Scholar]


