Abstract
Background
Thermal ablation for Barrett’s oesophagus has widely been established in gastrointestinal endoscopy during the last decade. The mainly used methods of radiofrequency ablation (RFA) and argon-plasma coagulation (APC) carry a relevant risk of stricture formation of up to 5–15%. Newer ablation techniques that are able to overcome this disadvantage would therefore be desirable. The aim of the present study was to compare the depth of tissue injury of the new method of Hybrid-APC versus standard APC within a randomized study in a porcine oesophagus model.
Methods
Using a total of eight explanted pig oesophagi, 48 oesophageal areas were ablated either by standard or Hybrid-APC (APC with prior submucosal fluid injection) using power settings of 50 and 70 W. The depth of tissue injury to the oesophageal wall was analysed macroscopically and histopathologically.
Results
Using 50 W, mean coagulation depth was 937 ± 469 µm during standard APC, and 477 ± 271 µm during Hybrid-APC (p = 0.064). Using 70 W, coagulation depth was 1096 ± 320 µm (standard APC) and 468 ± 136 µm (Hybrid-APC; p = 0.003). During all settings, damage to the muscularis mucosae was observed. Using standard APC, damage to the submucosal layer was observed in 4/6 (50 W) and 6/6 cases (70 W). During Hybrid-APC, coagulation of the submucosal layer occurred in 2/6 (50 W) and 1/6 cases (70 W). The proper muscle layer was only damaged during conventional APC (50 W: 1/6; 70 W: 3/6).
Limitations
Ex-vivo animal study with limited number of cases.
Conclusions
Hybrid-APC reduces coagulation depth by half in comparison with standard APC, with no thermal injury to the proper muscle layer. It may therefore lead to a lower rate of stricture formation during clinical application.
Keywords: Argon-plasma coagulation, Hybrid-APC, standard APC, submucosal injection
Introduction
Argon-plasma coagulation (APC) is a widely established ablation technique in gastrointestinal endoscopy.1–4 It has been shown to be effective and safe in various indications, such as thermal ablation of Barrett’s mucosa.1,2 and the treatment of vascular malformations.3,4
After endoscopic resection of early Barrett’s neoplasia, APC can be effectively used to ablate the remainder of the Barrett’s segment.5 Through this, the rate of metachronous neoplasia can significantly be reduced.6 The relatively newer technology of radiofrequency ablation (RFA)7 is now often chosen over APC for ablation, especially if longer Barrett’s segments are present. However, no trial comparing RFA with APC has yet been reported.
Both ablation techniques have several drawbacks. After APC, relevant rates of buried Barrett’s glands below the newly formed squamous epithelium have been reported, and those glands may carry a risk of malignant transformation.8 Relapse of Barrett’s mucosa has been reported after APC ablation.8 Potential complications of APC consist of stricture formation1 and, rarely, bleeding or perforation.9 In comparison with APC, RFA may allow quicker, more homogeneous and widespread ablation of Barrett’s mucosa, together with a favourably low rate of complications. Nevertheless, stricture formation is reported in about 5% of patients.7 It can therefore be assumed that there is still a need for technical improvement concerning Barrett’s ablation. An ideal technique would on the one hand lead to complete Barrett’s ablation, and on the other hand carry a very low risk of complications.
One possible approach to reduce the stricture rate may be submucosal injection of fluid prior to thermal ablation.10,11 Through this, injury to deeper layers of the oesophageal wall may be prevented.12–15 The so-called ‘Hybrid-APC’ is a newly developed technique that combines submucosal injection of isotonic sodium saline with standard APC in one instrument. However, its thermal effect on the oesophageal wall has not yet been assessed.
The aim of this randomized trial was to compare the depth of tissue injury after application of Hybrid-APC versus standard APC in a porcine oesophagus model as a potential basis for future clinical application.
Methods
Technical devices
A diagnostic gastroscope (GIF-Q145; Olympus Europe, Hamburg, Germany) was used for all procedures. As electrosurgical system, an APC generator (VIO 300D) and an APC2-unit (both Erbe Elektromedizin, Tuebingen, Germany) were used. This system was used for both the standard and the Hybrid-APC treatment.
For the new Hybrid-APC approach, the electrosurgical system was combined with a surgical water-jet system (ERBEJet2, same company) including a newly developed flexible water-jet tube for submucosal fluid injection. A flexible APC probe (20132-156; Erbe) with a diameter of 2.3 mm and a length of 2.2 m producing an axial plasma beam was combined with a flexible water-jet tube (Figure 1). The integrated water-jet nozzle had a diameter of 120 µm.
Figure 1.
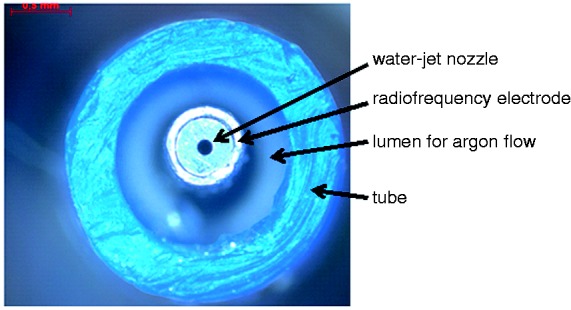
Top view of the distal part of the Hybrid-APC prototype.
During application of both methods, electrical settings for standard and Hybrid-APC were pulsed APC, effect 2, with a wattage of 50 and 70. The argon flow was constantly set at 0.9 l/min. For submucosal cushioning using the water-jet system, effect 60 was used.
Interventions
A total of eight porcine oesophagi were placed in an Erlangen Active Simulator for Interventional Endoscopy (EASIE). This simulator has been described in detail elsewhere.8 According to a computer-generated randomization list, six lesions were created in each oesophagus (total number of lesions n = 48). In the case of Hybrid-APC, lifting of the target area was performed by submucosal injection of 0.9% isotonic saline solution prior to APC (ERBEJet2, effect 60).
Effect 60 equates to a pressure of approximately 60 bar if a 120 µm water-jet nozzle (as applied in this study) is used. The procedure was standardized during all experiments. A total volume of 2 ml of fluid was applied per procedure. The duration of the injection was 2 seconds. The thickness of the submucosal cushion was 4–6 mm (macroscopic evaluation).
Both the standard APC flexible probe and the Hybrid-APC probe were applied at an estimated distance of 2 mm to the oesophageal wall (adjustment by the endoscopist; H.M.) for a duration of 4 seconds.
After ablation, the lesions were harvested for macroscopic and histopathological analysis. Of a total of 48 lesions, 24 were analysed microscopically, and the other 24 lesions were analysed macroscopically concerning coagulation diameter, coagulation depth, thermal injury to the proper muscle layer, and perforation. The coagulation depths were measured starting at the basement membrane because of the treatment-related destruction of the mucosal layer.
Histology
The samples were fixed on cork. They were then fixed in phosphate-buffered formalin (Rothifix 4.5%; Carl Roth, Karlsruhe, Germany), embedded in paraffin, cut in slices and stained with hematoxylin and eosin. Untreated tissue from the border of the thermal lesion was stained with hematoxylin and eosin to determine the thickness of the non-treated oesophagus.
Statistics
Estimation of number of cases
The depth of tissue injury was assumed to be up to 20% smaller after Hybrid-APC in comparison with standard APC without fluid cushioning. According to preliminary tests (data not shown), coagulation depth of standard APC (FORCED APC 70 W, argon flow 1 l/min, application time 5 s) was determined to be about 2.5 ± 0.4 mm. Assuming a difference of 20% between the groups with a significance level of 5%, a statistical power of 80%, and a drop-out rate of 10%, the total number of cases calculated was n = 12 per group.
Data were collected and analysed using descriptive statistics (means and standard deviations) and statistical hypothesis testing. Comparisons between groups were performed by single factor variance analysis and Tukey post-test. Normal distribution was evaluated by the Kolmogorov–Smirnov test. P-values less than 0.05 were considered statistically significant.
Results
Macroscopic investigation
An overview of the coagulation depths measured macroscopically is shown in Table 1. Figure 2 shows macroscopic examples of thermal damage to the oesophagus after standard APC with 70 W (Figure 2(a)) and Hybrid-APC with 70 W (Figure 2(b)). No perforation was observed. The area of coagulation was 41–56 mm2 at 50 W, and 51–82 mm2 at 70 W, with similar areas for standard and Hybrid-APC.
Table 1.
Macroscopically measured coagulation depths after standard and Hybrid-APC
| mean ± SD (mm) |
|||
|---|---|---|---|
| Wattage (W) | standard APC | Hybrid-APC | p |
| 50 | 3.4 ± 0.6 | 1.6 ± 0.3 | 0.0006 |
| 70 | 4.4 ± 0.4 | 2.2 ± 1.1 | <0.0001 |
Figure 2.
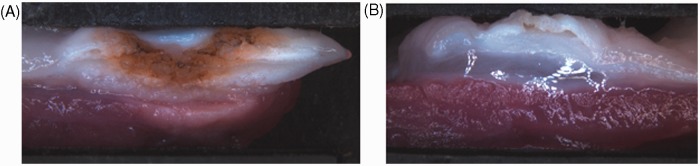
Macroscopically observed damage by standard (A) and Hybrid-APC (B), 70 W. (A) Damage to muscle layer can be observed macroscopically. (B) No thermal damage to muscle layer is observed after submucosal cushioning.
Microscopic investigation
Figure 3 gives an overview of the coagulation depths beyond the basement membrane of all four groups.
Figure 3.
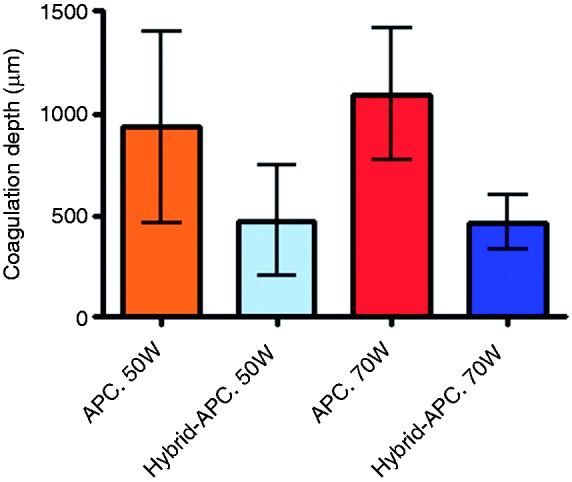
Histologically determined coagulation depth for standard and Hybrid-APC.
Using a power setting of 50 W, the mean coagulation depth beyond the basement membrane was 937 ± 469 µm during application of standard APC, and 477 ± 271 µm during Hybrid-APC (p = 0.064). Using 70 W, the mean coagulation depth was 1096 ± 320 µm (standard APC) and 468 ± 136 µm (Hybrid-APC; p = 0.003).
In Table 2, the damage to the different layers of the oesophageal wall after standard and Hybrid-APC is shown. In Figure 4, the damage to the different layers of the oesophageal wall after standard and Hybrid-APC is shown for 50 W (Figure 4(a)) and 70 W (Figure 4(b)).
Table 2.
Damage to oesophageal wall layers after standard and Hybrid-APC
| Randomization number | Randomization group | Epithelial layer | Lamina propria | Muscularis mucosae | Submucosa | Proper muscle layer |
|---|---|---|---|---|---|---|
| 50 W | ||||||
| 18 | APC | X | X | X | X | |
| 21 | APC | X | X | X | X | X |
| 29 | APC | X | X | X | ||
| 34 | APC | X | X | X | X | |
| 37 | APC | X | X | X | ||
| 43 | APC | X | X | X | X | |
| 17 | Hybrid-APC | X | X | X | ||
| 22 | Hybrid-APC | X | X | X | X | |
| 30 | Hybrid-APC | X | X | X | ||
| 33 | Hybrid-APC | X | X | X | ||
| 38 | Hybrid-APC | X | X | X | ||
| 44 | Hybrid-APC | X | X | X | X | |
| 70 W | ||||||
| 3 | APC | X | X | X | X | |
| 9 | APC | X | X | X | X | X |
| 13 | APC | X | X | X | X | X |
| 19 | APC | X | X | X | X | |
| 31 | APC | X | X | X | X | X |
| 46 | APC | X | X | X | X | |
| 4 | Hybrid-APC | X | X | X | X | |
| 10 | Hybrid-APC | X | X | X | ||
| 14 | Hybrid-APC | X | X | X | ||
| 20 | Hybrid-APC | X | X | X | ||
| 32 | Hybrid-APC | X | X | X | ||
| 45 | Hybrid-APC | X | X | X |
Figure 4.
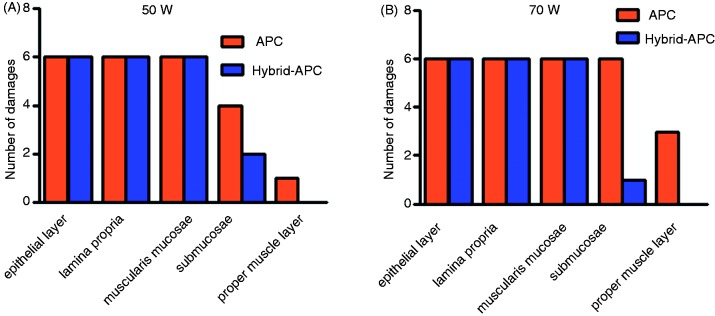
Damage to oesophageal wall layers by standard and Hybrid-APC, 50 W (A) and 70 W (B).
During all four settings, damage to the muscularis mucosae was observed. Using standard APC, damage to the submucosal layer was observed after 4/6 cases (50 W) and 6/6 cases (70 W). During Hybrid-APC, submucosal damage occurred in 2/6 cases (50 W) and 1/6 cases (70 W).
The proper muscle layer was only damaged during standard APC (50 W 1/6; 70 W: 3/6). Figure 5 shows histological sections of cases with a visible damage to the proper muscle layer. Figure 6 shows a control section.
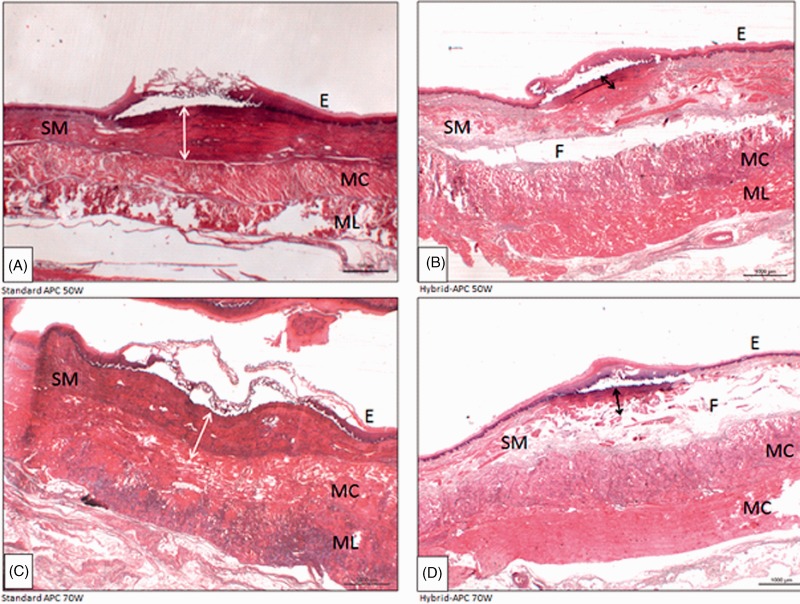
Figure 5.
Histological sections demonstrating the depth of thermal damage (hematoxylin-eosin) by standard (A, C) and Hybrid-APC (B, D). (A) Standard APC 50W: damage to E and SM, bordering the MC (B) Hybrid-APC 50W: damage to E and partly to SM. Protective fluid cushion can be seen. (C) Standard APC 70W: damage reaching MC. (D) Hybrid-APC, 70W: damage reached SM. E = squamous epithelium; SM = submucosal layer; MC = circular muscle layer; ML = longitudinal muscle layer; F = fluid cushion (Hybrid-APC).
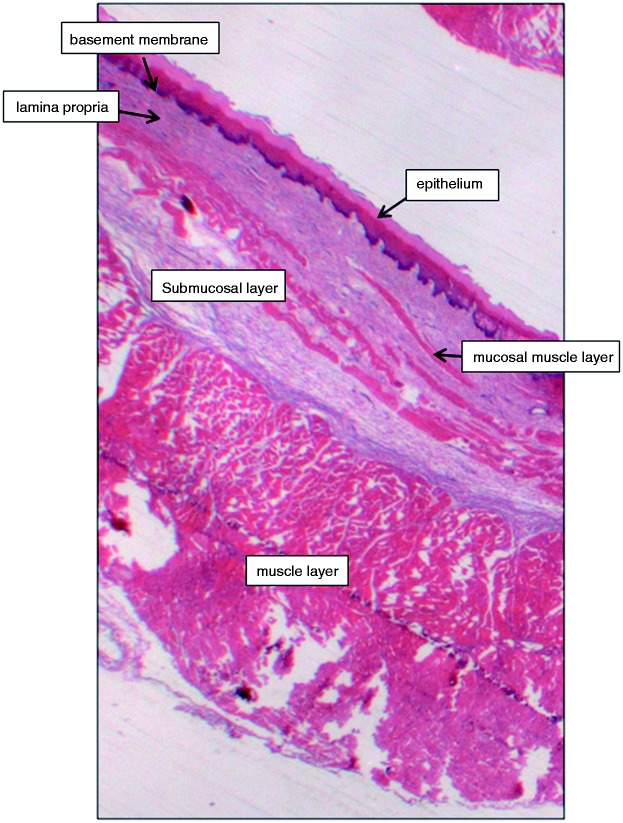
Figure 6.
Histological control sections section of a normal pig oesophagus, demonstrating the relevant layers of the oesophageal tissue.
The thickness of the submucosal cushion was not histologically determinable because of the fluid accumulated in the submucosal layer during fixation with formalin overnight. A remaining cushion of 700 µm on average was observed during histological assessment.
Discussion
During recent years, various tools for ablation of Barrett’s oesophagus have been reported; APC and RFA are widely used for this purpose.1–4,6–9
The ideal ablation technology has not yet been introduced to clinical practice. The two most established methods of APC and RFA carry a relevant risk of stricture formation of 5–15%.1–4,6–9 In addition, buried glands as well as incomplete ablation of Barrett’s mucosa or even relapse after initially complete ablation may occur.8 Therefore, further innovation concerning Barrett’s ablation is desirable.
During the present study, the depth of tissue injury of a newly developed ablation technology, the so-called ‘Hybrid-APC’, was evaluated within a randomized ex-vivo trial. The coagulation depth achieved was compared with standard APC, which is usually carried out without prior submucosal injection.
The rationale for the development of Hybrid-APC was the idea that fluid cushioning prior to APC may lead to a lower rate of complications during clinical application, especially stricture formation.10–12 First experiences on the use of a fluid cushioning before APC treatment were reported by Fujishiro and co-workers in 2006.11 However, the Hybrid-APC combination instrument had not yet been developed at that time.
The results of our study show that using Hybrid-APC, the coagulation depth can be reduced by half in comparison with standard APC. Using the relatively high wattage of 70 W, this difference was statistically significant.
During application of Hybrid-APC, coagulation damage to the submucosal layer was found in only the minority of applications. In contrast, with standard APC the submucosal layer was thermally damaged in more than half of applications using 50 W and in all cases using 70 W. In addition, the proper muscle layer was harmed in the standard APC group, but not in the Hybrid-APC group. It can therefore be assumed that Hybrid-APC may lead to a lower rate of stricture formation during clinical application. However, this question can only be answered within a clinical trial.
Interestingly, it has not yet been clarified if treatment-related stricture formation in the oesophagus only arises because of damage to the submucosal layer with consecutive fibrosis, or also because of damage to the proper muscle layer. Stricture formation after not only thermal ablation, but also radical endoscopic resection of early Barrett’s neoplasia, is a well-known complication of endoscopic therapy.16 In these cases, the main tissue damage is found at the level of the submucosa, where the cutting and coagulation current is applied.
The question arises whether Hybrid-APC may lead to a lower number of treatment sessions required for complete Barrett’s ablation. However, this question can only be answered within clinical trials. Further clinical advantages of Hybrid-APC may consist of its relatively easy application without the need for additional equipment, such as sizing balloons which are used prior to RFA in the tubular oesophagus. On the other hand, the use of APC in the oesophagus may in general be more operator-dependent than RFA, because the APC probe has to be positioned exactly and dynamically by the endoscopist in order to obtain a homogenous ablation effect.
Our results suggest that during clinical application of Hybrid-APC, all Barrett’s mucosa may be effectively ablated. Histopathologically, the muscularis mucosae was always damaged. This was independent of the APC technique used and of the power setting chosen (50 or 70 W). Because of the artificial thickening of the submucosal layer by fluid, relatively high power settings of 60–70 W may be used for clinical application of Hybrid-APC in order to guarantee a high eradication effect.
Interestingly, the mean coagulation depth achieved by Hybrid-APC with 70 W (477 microns) was similar to the coagulation depth achieved using 50 W (468 microns). Using standard APC, the coagulation depth only increased slightly from 937 to 1096 microns. A potential explanation for this may be the high electrical resistance of ex-vivo porcine tissue. In addition, the surface of the target tissue desiccates very quickly during APC, leading to an even higher resistance. Because of this, an increase in the wattage may in general lead to only small differences in the coagulation depth. It has to be remarked that during clinical application, another important factor influencing the coagulation depth is the activation time of APC.
The question arises whether it would have been useful to choose an APC mode other than ‘pulsed APC’ for the present study. It is known that the various APC modes available (pulsed, forced, and precise APC) have different coagulation effects.2,15,17,18 ‘Forced APC’ is characterized by a continuous energy output and thus by a strong coagulation and haemostatic effect. In contrast to ‘forced APC’, the application of energy with ‘pulsed APC’ is intermittent (pulsed), and this mode shows a constant voltage over the whole setting range. ‘Precise APC’ is used in thermally sensitive regions and if no strong power or haemostatic capability is needed. For the present study, we decided to use ‘pulsed APC’, because this mode generates a more homogeneous tissue effect in comparison with ‘forced APC’. In addition, less superficial carbonization is observed. ‘Precise APC’ cannot deliver enough energy for Barrett’s ablation.
The present study has several limitations. First, it is an ex-vivo animal study. In living animals, the coagulation depth observed after application of standard and Hybrid-APC may differ from the values measured here, because of the different physical properties of the tissues. Second, experience from animal models can be transferred to human tissue only to a limited extent. Third, only a limited number of applications were used for this study. However, this is the first trial on the application of Hybrid-APC that has been reported to date. Fourth, no comparison with the main clinical competitor RFA was performed during the present study. However, we decided to compare the two methods of APC as a first step. As a second step, a trial comparing Hybrid-APC to RFA would be helpful. Finally, a relatively high effect setting of 60 was used for submucosal cushioning during the present trial. This derives from the fact that pig oesophagi have a higher tissue resistance in comparison with human oesophagi and therefore require relatively higher power for injection.
Despite all enthusiasm for the new ablation technique of Hybrid-APC, the question arises whether its clinical use may lead to a relatively higher rate of buried glands in comparison with RFA or even standard APC. According to the present results, the coagulation depth of Hybrid-APC did not exceed a mean of 500 µ. In contrast, standard APC led to a coagulation depth of approximately 1000 µ. However, it should be kept in mind that the results of the present study can be transferred to clinical application only to a limited extent. The physical properties of living human tissue are different to those in an ex-vivo porcine oesophagus model. The electrical resistance of living tissue is likely to be lower because of a higher content of fluid inside the different layers of the oesophageal wall. A relatively higher coagulation depth may therefore be achieved during clinical application of Hybrid-APC, leading to destruction of buried Barrett’s glands in the lamina propria of the mucosal layer. Again, only a clinical trial evaluating the presence of buried glands after Hybrid-APC for Barrett’s ablation will be able to answer this question.
It is also unclear whether the injection of a type of fluid other than sodium chloride would lead to a more homogenous ablation effect or to a different coagulation depth.13 During the present trial, sodium chloride at a dilution of 0.9% was used for submucosal cushioning.
In conclusion, Hybrid-APC appears to be able to reduce the coagulation depth by half in comparison with standard APC, with no thermal injury to the proper muscle layer. It may therefore lead to a lower rate of stricture formation during Barrett’s ablation.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
Alexander Neugebauer and Markus D. Enderle are employees of Erbe Elektromedizin, Tuebingen, Germany. The other authors declare to have nothing to disclose.
Authors’ contributions
Conception and design: Hendrik Manner, Markus D. Enderle; analysis and interpretation of the data: Hendrik Manner, Marcus Scharpf, Kirsten Braun; drafting of the article: Hendrik Manner, Alexander Neugebauer; critical revision of the article for important intellectual content: Christian Ell, Falko Fend, Markus D. Enderle; final approval of the article: Hendrik Manner, Markus D. Enderle
References
- 1.Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett’s mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): A prospective multicenter evaluation. Am J Gastroenterol 2006; 101: 1762–1799 [DOI] [PubMed] [Google Scholar]
- 2.Manner H, May A, Rabenstein T, et al. Prospective evaluation of a new high-power argon plasma coagulation system (hp-APC) in therapeutic gastrointestinal endoscopy. Scand J Gastroenterol 2007; 42: 397–405 [DOI] [PubMed] [Google Scholar]
- 3.May A, Friesing-Sosnik T, Manner H, et al. Long-term outcome after argon-plasma coagulation of small-bowel lesions using double-balloon enteroscopy in patients with mid-gastrointestinal bleeding. Endoscopy 2011; 43: 759–765 [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc 2012; 24: 237–242 [DOI] [PubMed] [Google Scholar]
- 5.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008; 57: 1200–1206 [DOI] [PubMed] [Google Scholar]
- 6.Manner H, Rabenstein T, Pech O. Ablation of remaining Barrett’s epithelium after endoscopic resection: A randomized long-term follow-up study on argon plasma coagulation versus surveillance (APE study). Endoscopy 2014; 46: 6–12 [DOI] [PubMed] [Google Scholar]
- 7.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees JR, Lao-Sirieix P, Wong A, et al. Treatment for Barrett’s esophagus. Cochrane Database Syst Rev 2010; 1: CD004060–CD004060 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Manner H. Argon plasma coagulation therapy. Curr Opin Gastroenterol 2008; 24: 612–616 [DOI] [PubMed] [Google Scholar]
- 10.Fujishiro M, Kodashima S, Ono S, et al. Submucosal injection of normal saline can prevent unexpected deep thermal injury of argon plasma coagulation in the in vivo porcine stomach. Gut Liver 2008; 2: 95–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujishiro M, Yahagi N, Nakamura M, et al. Submucosal injection of normal saline may prevent tissue damage from argon plasma coagulation: An experimental study using resected porcine esophagus, stomach, and colon. Surg Laparosc Endosc Percutan Tech 2006; 16: 307–311 [DOI] [PubMed] [Google Scholar]
- 12.Norton ID, Wang L, Levine SA, et al. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc 2002; 56: 95–99 [DOI] [PubMed] [Google Scholar]
- 13.Sold MG, Grobholz R, Post S, et al. Submucosal cushioning with water jet before endoscopic mucosal resection: Which fluids are effective? Surg Endosc 2008; 22: 443–447 [DOI] [PubMed] [Google Scholar]
- 14.Kähler GF, Sold MS, Post S, et al. Selective tissue elevation by pressure injection (STEP) facilitates endoscopic mucosal resection (EMR). Surg Technol Int 2007; 16: 107–112 [PubMed] [Google Scholar]
- 15.Tsiamoulos ZP, Bourikas LA, Saunders BP. Endoscopic mucosal ablation: A new argon plasma coagulation/injection technique to assist complete resection of recurrent, fibrotic colon polyps (with video). Gastrointest Endosc 2012; 75: 400–404 [DOI] [PubMed] [Google Scholar]
- 16.Van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: A multicentre randomised trial. Gut 2011; 60: 765–773 [DOI] [PubMed] [Google Scholar]
- 17.Eickhoff A, Jakobs R, Schilling D, et al. Prospective nonrandomized comparison of two modes of argon beamer (APC) tumor desobstruction: Effectiveness of the new pulsed APC versus forced APC. Endoscopy 2007; 39: 637–642 [DOI] [PubMed] [Google Scholar]
- 18.Eickhoff A, Enderle MD, Hartmann D, et al. Effectiveness and safety of PRECISE APC for the treatment of bleeding gastrointestinal angiodysplasia – a retrospective evaluation. Z Gastroenterol 2011; 49: 195–200 [DOI] [PubMed] [Google Scholar]