Abstract
Dysphagia is a common reason for referral for investigations of oesophageal motility. Impedance measurement has now been incorporated into commercially available diagnostic manometry systems for more than a decade. This innovation, which offered the ability to record patterns of bolus transport without the need for simultaneous radiology, has for the most part failed to live up to expectations, offering few additional diagnostic insights.
This review examines the potential pitfalls related to how impedance patterns are currently analysed and introduces and discusses the new concept of pressure-flow analysis integrating pressure and impedance measurements to derive new metrics linked to the pressures occurring within and around the bolus as it is being transported.
Keywords: Oesophageal motility, dysphagia, manometry, impedance, diagnosis
Introduction
Dysphagia is a common reason for referral for investigations of oesophageal motility. High-resolution manometry (HRM) with application of the Chicago Classification algorithm is now the investigative method of choice for the diagnosis of oesophageal motility disorders.1 However, standard measures of oesophageal function, be they based on traditional low-resolution pressure recordings or state-of-the art HRM, are normal in a considerable number of dysphagia patients, and they are poorly correlated to the subjective perception of swallowed boluses.2–4 Investigators often turn to simultaneous intraluminal impedance measurement to provide plausible explanations for patient symptoms based on evidence of abnormal bolus transport.
Impedance measurement has now been incorporated into commercially available diagnostic systems for more than a decade. This innovation offered the ability to record patterns of bolus transport in relation to oesophageal motor function without the need for simultaneous radiology. Considerable research has been undertaken aimed at determining whether impedance recordings add value to manometric testing of oesophageal motility. Studies have evaluated the effects of swallow interval,5 body posture,6,7 bolus consistency,6,8–11 bolus volume,11 meals,12 regional differences along the oesophagus13 and have established criteria for effective bolus clearance14 and normative values.15,16 Pathological correlates have been examined in relation to manometric sub-types of oesophageal motor dysfunction, such as ineffective oesophageal motility (IEM)17,18 and diffuse oesophageal spasm (DES),19 and/or in relation to the broad spectrum of non-obstructive dysphagia.4,20–23 Focused studies of clinical sub-populations have also been performed, including investigations of achalasia,24–27 scleroderma,27 reflux disease,28,29 rumination30 and obese patients.31 Finally, drug effects on bolus transit have also been investigated with pressure-impedance recording.3,19,32–36
It was hoped that the complementary information provided in relation to bolus transit would give further insights which may provide an explanation for symptoms, predict optimal therapy and/or detect vulnerability to, for example, dysphagia following fundoplication surgery. However, whilst allowing direct assessment of bolus transit and clearance, intraluminal impedance measurement has for the most part failed to live up to these early expectations, offering few additional diagnostic insights beyond those already provided by manometry alone.
The main focus of studies that have evaluated impedance measurement as an adjunct to manometry has been to compare patients within different manometric diagnoses and/or different symptom presentation profiles (e.g. chest pain vs. dysphagia). In patients with abnormal motor patterns, impedance measurement can define ‘effectiveness’, by showing whether bolus transit is normal despite the manometric pattern being abnormal (or vice versa). However, half of patients who report symptoms of dysphagia have normal bolus transit on impedance criteria, as do many patients with IEM (or weak peristalsis in HRM parlance).18,21,23 Hence the causes of symptoms in many patients remain unclear despite clarification of these functional aspects. In DES, patients with chest pain have been differentiated by having greater contractile pressures in association with normal bolus transit whilst DES patients with predominantly dysphagia symptoms tend to have failed bolus transit.19 Whilst these are interesting observations, such findings have low prognostic value and, more important, management based on this information has not been tested. Failed bolus transit is ubiquitous in association with scleroderma and achalasia.18,21 Indeed in achalasia significant pooling of bolus residues, particularly in the distal oesophagus, makes interpretation of impedance waveforms in the traditional way difficult due to low baselines and entrapment of air.25,37
The direct relationship between symptom intensity and impedance-based measurements of transit function has not been explored in detail. The available evidence is limited and contradictory. For example, failed bolus transit has been reported in relation to increased dysphagia symptoms in dysphagia patients with normal manometry38 and reflux patients with post-fundoplication dysphagia.39 However, swallow-by-swallow analyses have yielded no relationship between failed or delayed bolus transit on impedance measurements and the perception of bolus passage in controls3,40 and dysphagia patients.4 Two studies have examined pre- and postoperative dysphagia in reflux patients undergoing fundoplication29,41 and these did not report a relationship between delayed bolus transport on impedance and the presence of dysphagia symptoms.
Hence a causal association between measured dysfunction and symptomatology remains unclear. Motility and/or flow abnormalities may cause symptoms or may be merely epiphenomena. Patients with ‘normal’ findings may display hypersensitivity or hyper-vigilance, or may alternatively have oesophageal dysfunctions which are unrecognised due to the lack of test sensitivity. Whilst the technologies allowing measurement of pressure and impedance have become highly sophisticated (i.e. high-resolution impedance manometry, HRIM), the analysis of pressure-impedance recordings remains relatively simplistic and these issues are still unresolved.
Why the current impedance analysis paradigm fails
In order to understand why the addition of impedance analysis has failed to provide a substantial diagnostic gain over pressure-only analysis, it is important to examine the potential pitfalls related to how impedance patterns are currently analysed. This diagnostic paradigm is based on a simple dichotomous characterisation of complete vs. incomplete bolus clearance which is determined using a semi-automated analysis approach. Standard analysis of impedance recordings uses the 50% drop and recovery relative to baseline to define the timing of the arrival of the bolus head and the exit of the bolus tail, respectively. Failure of the impedance drop to recover to 50% defines transport failure within the relevant region of the oesophagus.
Whilst impedance measurements and barium swallow are concordant,23,33,37,42,43 the 50% criteria are nevertheless problematic. Firstly, this approach relies of the existence of a stable baseline. However, impedance baselines in the oesophagus are highly variable over time and are influenced by luminal air and residue accumulation from previous swallows, particularly in patients with scleroderma and achalasia.22 Secondly, software used for the analysis is applied in a semi-automated fashion whereby the analyst can manually change key landmarks. In the high-resolution context, impedance colour contour plots can give a visual representation of bolus presence. This approach was originally developed and optimised in relation to detection of post-swallow residues in pharyngeal dysphagia patients.44,45 However, in practise this approach is routinely applied to oesophageal recordings through arbitrary manual adjustment of contour plot settings. Therefore, analysis of impedance waveforms is heavily dependent of subjective interpretations and personal preferences of the analyst. Finally, the main diagnostic outcome of the analysis is to dichotomously conclude complete or incomplete bolus clearance. Hence this standard approach only subjectively defines the presence of bolus transport failure and does not objectively quantify the degree and/or extent of bolus retention in relation to failure.
Simultaneous pressure-impedance measurement is now a widely available standard add-on to all state-of-the art motility systems (HRIM). However, the manometric diagnosis is paramount and impedance-based findings are secondary and interpreted in isolation through the prism of pressure-based findings of a normal or disordered motility pattern. It is notable that in the modern era of high-resolution solid state manometry, with diagnosis using oesophageal pressure topography (EPT) metrics and the Chicago classification of oesophageal motor disorders, impedance measurement does not currently feature in the classification.1 This is largely because bolus transport failure, the main diagnostic outcome of impedance measurement, can be inferred when large peristaltic breaks are observed.46,47 Additionally, bolus stasis is frequently observed in controls and therefore is not pathological when considered in isolation.48
A clinically useful motility investigation should be able to distinguish patients with hypersensitivity from patients with true motor dysfunctions. Whilst studies have linked chest pain with hypertensive motor disorders,19 the association of heightened perception of swallowed boluses and other types of motor abnormalities is less clear. A firmer understanding of how specific oesophageal motor dysfunctions can lead to symptoms is needed. And the fact that current pressure or impedance-based variables do not reproducibly correlate with the subjective swallow-by-swallow perception of bolus hold-up in patients with dysphagia symptoms2,4 exposes a fundamental weakness in current diagnostic testing for oesophageal motor disorders.
It is important to recognise that the pressures in and around the bolus being transported may have physiological and pathophysiological relevance. However, current analysis approaches focus almost exclusively on the isometric tension generated by the circular muscle following lumen occlusion. Intrabolus distension pressures can be visualised in obstructive pathology as distal compartmentalised pressure in, for example, oesophagogastric junction (EGJ) outflow obstruction.1,49 However, intrabolus pressures are difficult to measure objectively, as are the pressures associated with muscle shortening before the lumen occludes and the circular muscle squeezes the catheter. These pressures are functionally important as they are primarily the mechanism responsible for bolus propulsion by peristalsis.
Potential new paradigms integrating pressure and impedance measurement
We argue that the current paradigm dictating how impedance patterns are analysed requires revision as does the overall approach of analysing impedance waveforms in isolation. Used in combination with pressure measurement, impedance measurement can guide derivation of additional functional variables which provide information in relation to intrabolus distension pressures, bolus-driving pressures and the degree and extent of clearance failure. Conversely, pressure measurements, which determine anatomical landmarks and lumen occlusion in space and time, can define where impedance should be measured to maximise reliability. This approach, called Pressure Flow Analysis (FPA), has now been applied to both pharyngeal and oesophageal swallow recordings from controls and patients.41,50–60 In practise, PFA is performed in an entirely automated, software-driven fashion whereby functional metrics are algorithmically derived following user selection of space-time landmarks which define regions of interest (ROI) on a standard Clouse EPT plot. Automated impedance manometry (AIM) software (called ‘AIMplot’) has been developed to enable PFA. Using AIMplot, and after a short training session, analysts of varying expertise can objectively derive pressure-flow metrics with high inter- and intra-rater reproducibility.61,62
At this juncture it is informative to re-evaluate the seminal biomedical studies of Silney and colleagues,43 who were the first to apply multi-channel intraluminal impedance to the measurement of gastrointestinal motility. The physiological factors that determine the level of impedance measured at any time or position along the oesophagus are complex, being influenced by the conductivities of luminal contents, layers of the oesophageal wall, and extra-oesophageal fluids and tissues. An important tenet for using intraluminal impedance to identify flow is use of a bolus that is substantially more conductive than the oesophageal wall, hence the reason why saline (0.9% NaCl) is typically used as a test bolus.
A less well-recognised feature of the impedance waveform is that, during passage of a highly conductive bolus, the lowest level of impedance recorded (i.e. nadir impedance) corresponds to the time and position where the lumen in most conductive; in normal circumstances this identifies the axial centre, or most distended part, of the intrabolus domain during transport43 (Figure 1). The measured value of nadir impedance has been shown to be an inverse correlate of maximum luminal diameter or cross-sectional area.63–65
Figure 1.
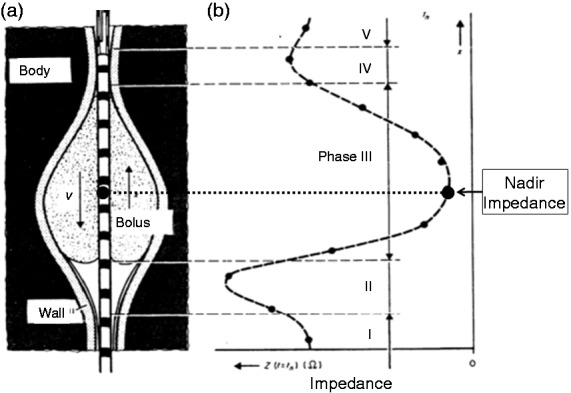
Estimation of the impedance changes caused by a bolus with air in front of it moving along the oesophagus. (a) Illustration of bolus shape and movement. (b) Spatial distribution of impedance along the catheter. Interpolation between discrete impedance values is shown as dotted lines. The nadir level of impedance corresponds approximately to the position of maximum bolus accumulation (axial centre). Drawing adapted from Silny et al.43 (1993) modified for purposes of simplification.
By identifying the axial centre of the bolus, the nadir impedance can be used to determine the trajectory of the bolus in time and space as it is propelled from the pharynx to the proximal oesophagus, down to the distal oesophagus and then through into the stomach via the EGJ. It has been long recognised that pharyngeal swallow can propel boluses significant distances along the length of the oesophagus. This aspect of swallowing physiology, originally described by Kronecker and Meltzer66 and documented scintigraphically by Buthpitiya et al.,67 has been largely ignored in the context of the potential assistance pharyngeal propulsion may give to bolus transport. The bolus trajectory pathway based on the time and location of nadir impedance shows a typical pattern of bolus deceleration followed by stasis (at the point of inflexion) and then acceleration. The bolus trajectory curve best-fit allows the flow stasis point (FSP) to be determined objectively.55 The FSP most likely represents the timing and location of the functional change from pharyngeal-driven bolus transport to oesophageal peristalsis-driven bolus transport, and hence may allow easier delineation of the different roles the pharynx and oesophagus play in bolus propulsion (Figure 2). Viscous boluses that are more resistant to movement and therefore harder to propel are not carried as far by pharyngeal propulsion alone and this places greater demand on oesophageal peristalsis to transport the bolus over a longer distance from the point of stasis to EGJ, increasing the likelihood of transport failure in relation to the presence of peristaltic breaks.59
Figure 2.
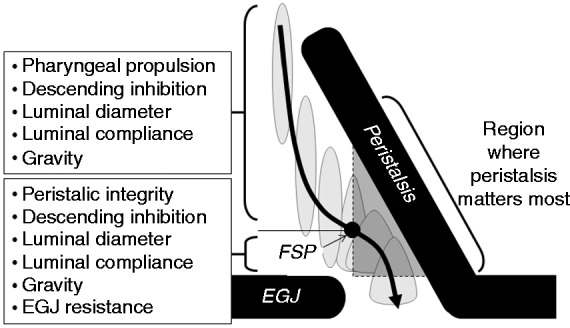
Determinants of the bolus trajectory pathway and resultant intrabolus pressures. Adapted from Omari et al.55 (2012).
FSP: flow stasis point.
There is growing recognition of the link between pharyngeal dysphagia and oesophageal motor dysfunction. A high proportion of pharyngeal dysphagia patients have peristaltic abnormalities of varying severity (personal communication 2014, I Cook, University of New South Wales) and pharyngeal abnormalities on modified barium swallow have been shown to correlate with oesophageal bolus transport abnormalities based on conventional impedance analysis.68 This highlights the fact that optimal assessment of dysphagia patients should preferably include measurements from both the pharynx and oesophagus. By uniquely integrating pharyngeal bolus propulsion with oesophageal bolus transport, determination of the FSP may prove useful, but this requires further studies.
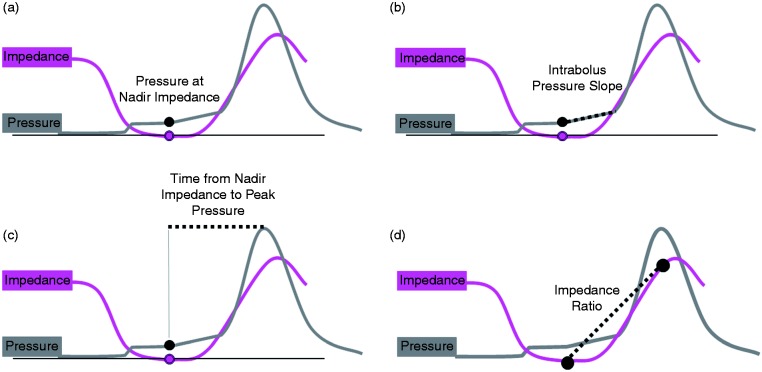
Intrabolus pressure (IBP) is a key manometric correlate of obstruction; however, IBPs can be highly variable in real time and IBPs are notoriously challenging to measure objectively. Hence IBPs are largely ignored unless unequivocally abnormal (e.g. distal compartmentalised pressures). It has been suggested,41,59 and now confirmed,65 that nadir impedance defines maximum bolus distension in space and time. Nadir impedance therefore provides a useful objective landmark for measurement of intrabolus distension pressures. Hence pressure at nadir impedance (PNadImp) defines intrabolus distension pressures during bolus transport (Figure 3(a)).
Figure 3.
Pressure flow analysis using automated impedance manometric (AIMplot) software. (a) Pressure at nadir impedance (PNadImp) defines intrabolus distension pressures during bolus transport. (b) Intrabolus pressure slope (IBPslope) can serve as a marker for the degree of pressurisation need to propel the bolus onward. (c) The time from nadir impedance to peak pressure (TNadImp to PeakP) is a marker of how far ahead of the peristaltic wave the bolus moving. (d) Impedance ratio is a marker of clearance.
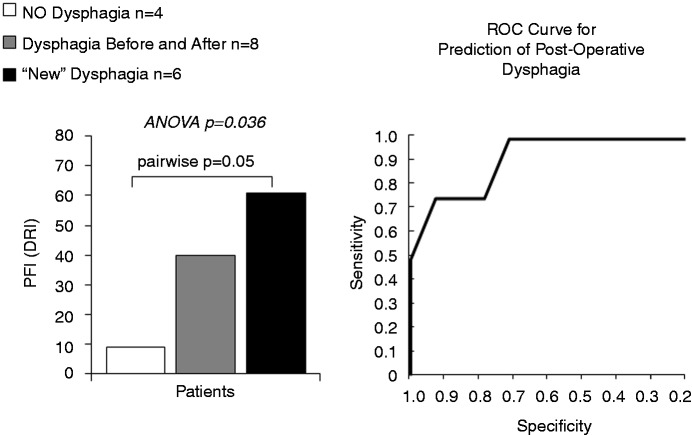
As part of normal physiology of bolus transport, maximal bolus distension is followed by circular muscle contraction which causes the lumen to narrow and occlude. The nadir impedance and peak pressure demarcate the limits over which active circular muscle contraction occurs. Within this region is a propulsive zone where muscle contraction and luminal emptying overlap prior to luminal occlusion which serves to seal the lumen, preventing retrograde bolus escape. The rate of pressure change within this propulsive zone, i.e. intrabolus pressure slope (IBP slope), can serve as a marker for the degree of pressurisation needed to propel the bolus onward (Figure 3(b)). The time from nadir impedance to peak pressure (TNadImp-PeakP) is also a marker of how far ahead of the peristaltic wave the bolus centre is located (Figure 3(c)). In addition to these individual metrics a pressure-flow index (PFI) has been empirically derived to serve as a global measure of pressure-flow abnormality. Based on the first studies that applied PFA to oesophageal recordings in reflux patients, the PFI (originally called the Dysphagia Risk Index) was found to be a pre-operative predictor of post-operative dysphagia41,58 (Figure 4).
Figure 4.
Pressure Flow Index (Dysphagia Risk Index) in the distal oesophagus based on viscous bolus swallows before surgery by dysphagia status after surgery. Data adapted from Myers et al.41 (2012).
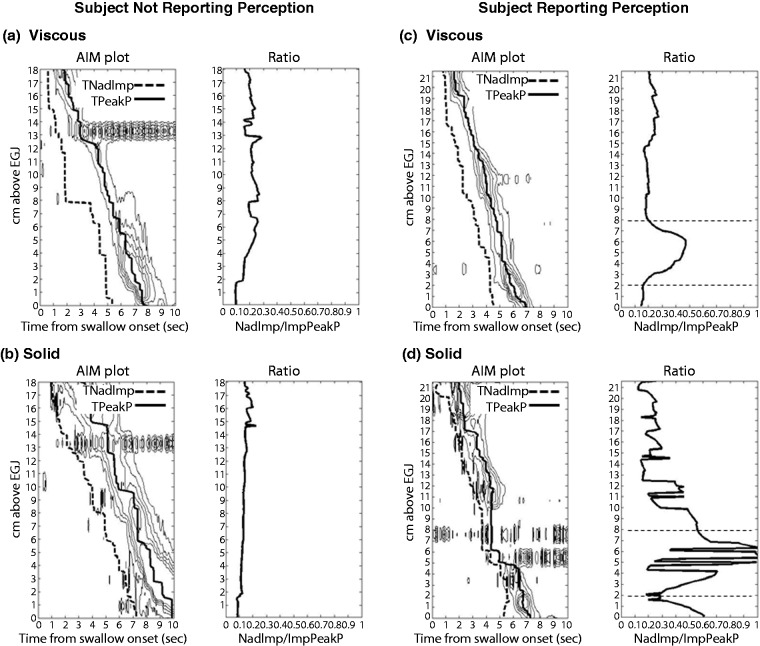
As previously mentioned, standard impedance analysis is problematic due to variability in the pre-swallow impedance which can fluctuate considerably due to the presence of air and the presence of bolus residue from previous swallows. There are now alternative methods to assess bolus clearance objectively that quantify the degree of clearance failure. To this end the concept of an Impedance Ratio (IR) has been proposed relating the nadir impedance (i.e. when the lumen is most distended/full) and the impedance measured at the peak of the esophageal peristaltic wave (i.e. when the lumen is most occluded/empty) (Figure 3(d)). Impedance ratio provides an objective and continuous measure influenced by both the extent bolus clearance failure along the length of the oesophagus as well the volume of residue retained (Figure 5). This method correlates with the standard method of impedance analysis,57,59 but may better allow pin-pointing of focally localised failure (Figure 5). Alternative approaches are the post-swallow pharyngeal integrated nadir impedance to impedance ratio (iZn/Z69) and the oesophageal impedance integral ratio (EII ratio70). All of these methods are similar in that they relate impedance measured before contraction to that after contraction and use pressure measurements to guide the positioning of the ROIs which define where the analysis is performed in space and time. These new methods of impedance analysis attempt to address the shortcomings of standard impedance analysis by objectively quantifying the degree and extent of clearance failure. However, it remains to be determined whether these methods can better differentiate pathological degrees of post-swallow bolus retention that lead to symptoms.
Figure 5.
Calculation of the impedance ratio along the oesophagus using automated impedance manometric (AIMplot) software. Example viscous and solid swallows are shown from a individual who reports no-bolus bolus perception ((a) and (b)) and an individual who perceives bolus passage for solids ((c) and (d)). Plots (left) show pressure contour plots (with iso-contours starting at 20 mmHg) with time of nadir impedance (TNadImp) and time of peak pressure (TPeakP) superimposed. Graphs (right) show the impedance ratio. The participant with bolus perception reported bolus stepwise or slow passage during the solid swallow, suggesting that some stasis had occurred. For this subject, both viscous and solid swallows ((c) and (d)) demonstrate a focal region of higher impedance ratio in the distal oesophagus between 2 cm and 8 cm above the oesophagogastric junction (EGJ) (this region is demarcated in graphs right by horizontal dotted lines). In the case of the solid swallow (d), a peristaltic break is also visible within this region, which would be compatible with incomplete bolus transit. From Omari et al.59 (2013). Reproduced with permission.
PFA approaches may provide insights into pathology in relation to swallowing disorders and the genesis of symptoms. Based on published studies, there is an emerging picture of this approach offering additional information complementary of a standard HRM diagnosis based on the Chicago Classification. Table 1 summarises four studies that have assessed changes in PFA metrics in relation to the perception of bolus transit.41,57,59,60 Of greatest interest in these early data is evidence that some metrics correlate with a heightened perception of bolus holdup and/or are altered in relation to vulnerability to dysphagia post-fundoplication surgery. All metrics did not correlate with symptoms in all studies. However, a higher Pressure Flow Index overall and an elevated IBP slope were the most reproducible findings in relation to symptom intensity.
Table 1.
Published findings of changes in pressure-flow metrics in relation to bolus perception
| Change in pressure flow metric (distal oesophagus) as related to subjective bolus perception | |||||||
|---|---|---|---|---|---|---|---|
| Publication | Method used | Cohort type | Bolus type | PNadIMP | IBPslope | Time NadImp to Peak P | PFI |
| Myers et al.41 2012 | 8-Channel perfusion | GERD | Viscous | NS | Increased | Shorter | Increased |
| Chen et al.57 2013 | 5-Channel solid state | NOD | Viscous | NS | Increased | NS | Increased |
| Omari et al.59 2013 | 32- to 36-Channel solid state | Control | Solid | NS | Increased | NS | Increased |
| Rommel et al.60 2014 | 21-Channel perfusion | Control | Solid | NS | Increased | NS | NS |
PNadIMP: pressure at nadir impedance; IBPslope: intrabolus pressure slope; Time NadImp to Peak P: time from nadir impedance to peak pressure; PFI: pressure-flow index; GERD: gastroesophageal reflux disease; NOD: non-obstructive dysphagia; NS: not statistically significant. Data shown for GERD patients relate to postoperative global dysphagia symptoms measured pre-operatively. Other data relate to perception on a swallow-by-swallow analysis.
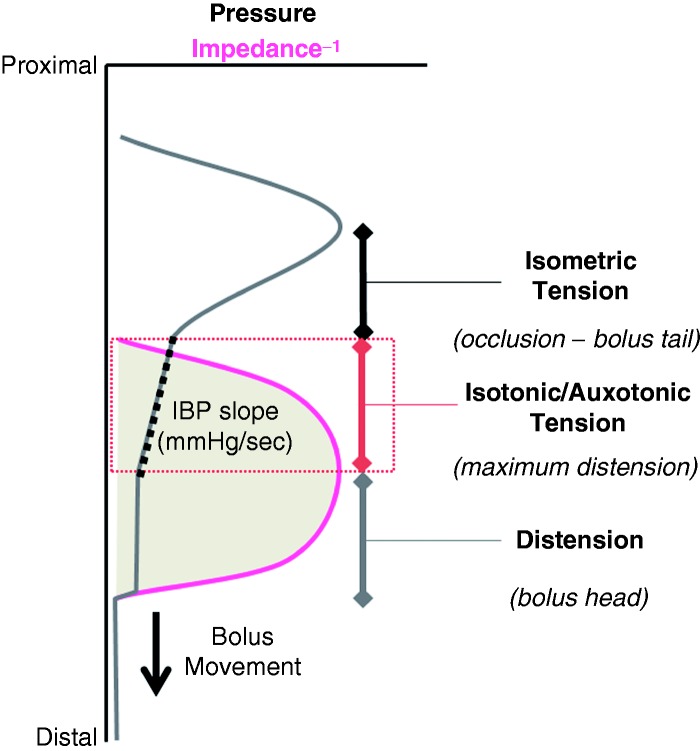
The observations in relation to IBP slope are interesting. This appears to give importance to the rate of tension generation between the phases of maximal distension and maximum lumen occlusion (Figure 6). This phase of contraction can be ‘isotonic’ (muscle shortening with no pressure increase) or ‘auxotonic’ (shortening with associate pressure increase).71,72 The apparent reproducibility of these early findings relating higher IBP slope to greater perception may provide an important clue to a pathological process. Indeed the mere existence of an auxotonic pressure profile (high IBP slope) suggests that the oesophagus is either working harder against increase bolus load because the bolus is of a less moveable consistency (solid boluses show higher IBP slope), or that the bolus has encountered an area of high resistance to normal flow. Resistance could be overtly related to a disease process which causes reduced wall compliance (e.g. tissue remodelling in relation to esosinophilic oesophagitis (EoE)) or to reduced neural relaxation of the EGJ (e.g. achalasia). However (and as shown in Table 1), high IBP slope relating to symptom perception has been documented in dysphagia patients without achalasia, EJG abnormalities or EoE. This appears to suggest that more subtle factors, possibly impaired neutrally mediated relaxation of the oesophageal lumen ahead of the bolus, may also precipitate symptoms. Alternatively bolus emptying across the EGJ may be impaired even though measurements of EGJ relaxation pressures suggest otherwise. In addition, and regardless of what may be causing these subtle pressure phenomena, it also appears that the oesophageal sensory afferent pathway governing sensation of luminal contents within the distal oesophagus may be specifically sensitive to the build-up of tension that occurs during the auxotonic contraction and largely insensitive to the isometric contraction after the bolus has passed.
Figure 6.
Intrabolus pressure (IBP) slope relates to tension generated during muscle contraction that is within the propulsive zone between distension with bolus and tension following occlusion of the lumen. Muscle contraction can be isotonic (lumen closing with no pressure increase), auxotonic (lumen closing with pressure increasing) or isometric (pressure increasing after lumen has closed).
Future directions
Corroboration of abnormal IRP findings
Diagnosis of oesophageal motor disorders based on EPT metrics and the Chicago Classification algorithm represents a major advance in clinical applicability of HRM. However, a potential vulnerability of the Chicago Classification is that it critically relies on a single parameter of EGJ relaxation, the 4 sec Integrated Relaxation Pressure (IRP4), to diagnose achalasia and EGJ outflow obstruction.1 The EGJ is a complex region demonstrating radial pressure asymmetries due to extrinsic structures73,74 and this complexity has been shown to produce erroneous recordings of high pressure within the EGJ when at the same time bolus flow across the EGJ is evident on radiology.75 The observation of a high EJG pressure is interpreted as representative of the restricted ability of contents to flow across the EGJ during deglutition. However, to our knowledge, published studies have not described a direct correlation between IRP4 and flow-time across the EGJ. Whilst conditions allowing bolus flow through the EGJ can be predicted based on the flow permissive time,75,76 the practicability of this technique is less certain because resolution of subtle pressure changes across the EGJ can be compromised by small drifts in pressure baselines. From the standpoint of a better assessment of EGJ function, impedance measurement may play a role in the future by providing direct evidence of flow across the EGJ,77 and PFA techniques may help to corroborate a diagnosis of EGJ outflow obstruction. During peristalsis, the region immediately proximal to the EGJ can be manometrically demarcated by the position of the contractile deceleration point.78 As this region is anatomically less complex than the EGJ, pressure-flow measures may allow inference of EGJ flow resistance.
Quantification of active inhibition
An inhibitory-excitatory imbalance has been implicated in the aetiology of primary oesophageal motor disorders and provocative methods such as multiple rapid swallow (MRS) have been proposed for indirect detection of impaired oesophageal inhibition during swallowing.79 Direct quantification of inhibitory mechanisms has not been possible based on intraluminal manometry alone. Intraluminal ultrasound has been successfully used to define a wave of active relaxation that precedes peristalsis;80 however, this elegant technique has limited spatial resolution and requires complex technology and measurement. It may also be possible to quantify the degree of neutrally mediated active and passive contraction and relaxation that is occurring in real time within muscle during peristalsis using combined spatiotemporal mapping of changes in intraluminal pressure and diameter.71,72,81 An extension of this novel approach is to use impedance change to infer diameter change. Proof of concept for this method has recently been achieved in the in vitro colon.71 This same technique may have the potential to directly quantify active inhibition of oesophageal circular muscle during swallowed bolus transport and this may have utility for understanding oesophageal physiology and pathophysiology. However, developing such predictive models will be more challenging in the in vivo setting. The relationship between luminal area change and corresponding impedance change is more complex to model in vivo because the conductivity of the bolus alone is responsible for most of the impedance change that occurs when bolus material first enters the lumen.65
Guiding therapy
Current therapies for dysphagia are aimed at: i. stimulating contraction when motility is weak (e.g. cholinergic agonists, 5HT agonists), ii. reducing hypersensitivity when motility is normal (e.g. tricyclic antidepressants) or iii. attenuating contraction when motility is hypertensive (e.g. nitrates, Ca channel blockers, botulinum toxin injection and phosphodiesterase 5 inhibitors). Overall, such therapies have performed poorly in therapeutic trials. One potential reason for this lack of efficacy is that current treatments are poorly targeted due to lack of sensitivity of testing to identify if oesophageal dysmotility is causing symptoms. A further extension of the PFA paradigm, beyond examination of individual metrics in isolation, is to combine the Pressure Flow Index with impedance ratio to dichotomously separate out patients with dysphagia who have predominately abnormal bolus clearance and/or those with abnormal bolus flow resistance. Figure 7 illustrates this distribution within a cohort of patients with non-obstructive dysphagia.57 As shown in Figure 8, this type of diagnostic stratification may enable specific targeting of pharmacological therapies designed to improve bolus clearance or reduce flow resistance.
Figure 7.
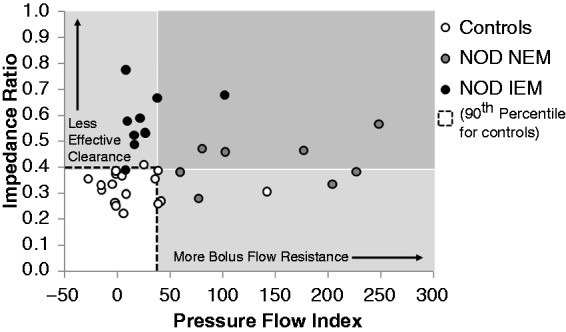
Categorisation of non-obstructive dysphagia patients based on Pressure Flow Index (PFI) and Impedance Ratio enables a dichotomous separation of patients who have predominately abnormal bolus clearance (mostly IEM) and/or those with abnormal bolus flow resistance (mostly Normal Esophageal Motility, NEM). Mean viscous bolus data from individual patients and controls are shown. The dotted box indicates the range determined for control participants. From Chen et al.57 (2013). Reproduced with permission.
Figure 8.
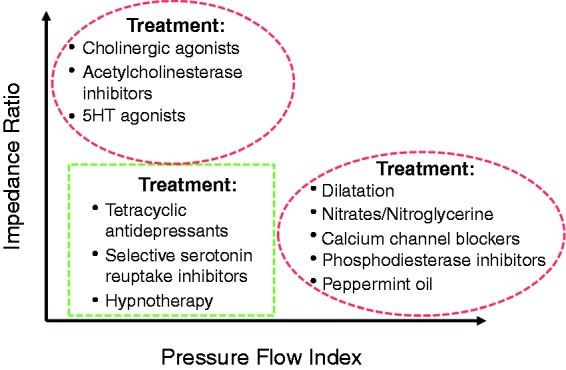
Model of how swallow categorisation based on Pressure Flow Index (PFI) and impedance ratio may potentially guide therapy.
Conclusion
Despite considerable investment of research effort, conventional methods of incorporation of impedance measurements into clinical diagnosis of oesophageal motility disorders have failed to produce a significant diagnostic dividend. PFA performed using automated impedance manometry is a new method for analysis of intraluminal pressure-impedance recordings, integrating pressure and impedance measurements to derive new metrics linked to the pressures occurring within and around the bolus as it is being transported. Early findings have reproducibly demonstrated an association between heighted bolus perception and PFA metrics. This contrasts with findings based on conventional impedance analysis and analysis of motor patterns based on isometric tension measurements.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
T Omari, N Rommel: patent on pressure flow analysis methods.
References
- 1.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil 2012; 24(Suppl 1): 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao Y, Kahrilas PJ, Nicodème F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol 2014; 109: 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarescu A, Karamanolis G, Aprile L, et al. Perception of dysphagia: Lack of correlation with objective measurements of esophageal function. Neurogastroenterol Motil 2010; 22: 1292–1297, e336–e337 [DOI] [PubMed] [Google Scholar]
- 4.Chen CL, Yi CH. Clinical correlates of dysphagia to oesophageal dysmotility: Studies using combined manometry and impedance. Neurogastroenterol Motil 2008; 20: 611–617 [DOI] [PubMed] [Google Scholar]
- 5.Tutuian R, Jalil S, Katz PO, et al. Effect of interval between swallows on oesophageal pressures and bolus movement in normal subjects – Studies with combined multichannel intraluminal impedance and oesophageal manometry. Neurogastroenterol Motil 2004; 16: 23–29 [DOI] [PubMed] [Google Scholar]
- 6.Bernhard A, Pohl D, Fried M, et al. Influence of bolus consistency and position on esophageal high-resolution manometry findings. Dig Dis Sci 2008; 53: 1198–1205 [DOI] [PubMed] [Google Scholar]
- 7.Tutuian R, Elton JP, Castell DO, et al. Effects of position on oesophageal function: Studies using combined manometry and multichannel intraluminal impedance. Neurogastroenterol Motil 2003; 15: 63–67 [DOI] [PubMed] [Google Scholar]
- 8.Chen CL, Szczesniak MM, Cook IJ. Identification of impaired oesophageal bolus transit and clearance by secondary peristalsis in patients with non-obstructive dysphagia. Neurogastroenterol Motil 2008; 20: 980–988 [DOI] [PubMed] [Google Scholar]
- 9.Chen CL, Yi CH. Assessment of esophageal motor function using combined multichannel intraluminal impedance and manometry in healthy volunteers: A single-center study in Taiwan. J Gastroenterol Hepatol 2007; 22: 1039–1043 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen HN, Domingues GR, Winograd R, et al. Impedance characteristics of normal oesophageal motor function. Eur J Gastroenterol Hepatol 2003; 15: 773–780 [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan R, Vela MF, Katz PO, et al. Esophageal function testing using multichannel intraluminal impedance. Am J Physiol Gastrointest Liver Physiol 2001; 280: G457–G462 [DOI] [PubMed] [Google Scholar]
- 12.Wilson JA, Mainie I, Tutuian R, et al. Multichannel intraluminal impedance and esophageal manometry data for unrestricted swallowing: Establishing normal values. Dis Esophagus 2008; 21: 51–56 [DOI] [PubMed] [Google Scholar]
- 13.Wise JL, Murray JA, Conklin JL. Regional differences in oesophageal motor function. Neurogastroenterol Motil 2004; 16: 31–37 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen NQ, Tippett M, Smout AJ, et al. Relationship between pressure wave amplitude and esophageal bolus clearance assessed by combined manometry and multichannel intraluminal impedance measurement. Am J Gastroenterol 2006; 101: 2476–2484 [DOI] [PubMed] [Google Scholar]
- 15.Tutuian R, Vela M, Nagammapudur S, et al. Esophageal function testing with combined multichannel intraluminal impedance and manometry: Multicenter study in healthy volunteers. Clin Gastroenterol Hepatol 2003; 1: 174–182 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen NQ, Rigda R, Tippett M, et al. Assessment of oesophageal motor function using combined perfusion manometry and multi-channel intra-luminal impedance measurement in normal subjects. Neurogastroenterol Motil 2005; 17: 458–465 [DOI] [PubMed] [Google Scholar]
- 17.Blonski W, Vela M, Safder A, et al. Revised criterion for diagnosis of ineffective esophageal motility is associated with more frequent dysphagia and greater bolus transit abnormalities. Am J Gastroenterol 2008; 103: 699–704 [DOI] [PubMed] [Google Scholar]
- 18.Tutuian R, Castell DO. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: Studies using combined impedance-manometry. Clin Gastroenterol Hepatol 2004; 2: 230–236 [DOI] [PubMed] [Google Scholar]
- 19.Tutuian R, Mainie I, Agrawal A, et al. Symptom and function heterogenicity among patients with distal esophageal spasm: Studies using combined impedance-manometry. Am J Gastroenterol 2006; 101: 464–469 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NQ, Ching K, Tippett M, et al. Impact of nadir lower oesophageal sphincter pressure on bolus clearance assessed by combined manometry and multi-channel intra-luminal impedance measurement. Neurogastroenterol Motil 2010; 22: 50–55, e9 [DOI] [PubMed] [Google Scholar]
- 21.Conchillo JM, Nguyen NQ, Samsom M, et al. Multichannel intraluminal impedance monitoring in the evaluation of patients with non-obstructive dysphagia. Am J Gastroenterol 2005; 100: 2624–2632 [DOI] [PubMed] [Google Scholar]
- 22.Cho YK, Choi MG, Park JM, et al. Evaluation of esophageal function in patients with esophageal motor abnormalities using multichannel intraluminal impedance esophageal manometry. World J Gastroenterol 2006; 12: 6349–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: Study in 350 patients. Am J Gastroenterol 2004; 99: 1011–1019 [DOI] [PubMed] [Google Scholar]
- 24.Tatum RP, Wong JA, Figueredo EJ, et al. Return of esophageal function after treatment for achalasia as determined by impedance-manometry. J Gastrointest Surg 2007; 11: 1403–1409 [DOI] [PubMed] [Google Scholar]
- 25.Conchillo JM, Selimah M, Bredenoord AJ, et al. Assessment of oesophageal emptying in achalasia patients by intraluminal impedance monitoring. Neurogastroenterol Motil 2006; 18: 971–977 [DOI] [PubMed] [Google Scholar]
- 26.Agrawal A, Hila A, Tutuian R, et al. Manometry and impedance characteristics of achalasia. Facts and myths. J Clin Gastroenterol 2008; 42: 266–270 [DOI] [PubMed] [Google Scholar]
- 27.Mainie I, Tutuian R, Patel A, et al. Regional esophageal dysfunction in scleroderma and achalasia using multichannel intraluminal impedance and manometry. Dig Dis Sci 2008; 53: 210–216 [DOI] [PubMed] [Google Scholar]
- 28.Del Genio G, Tolone S, Del Genio F, et al. Impact of total fundoplication on esophageal transit: Analysis by combined multichannel intraluminal impedance and manometry. J Clin Gastroenterol 2012; 46: e1–e5 [DOI] [PubMed] [Google Scholar]
- 29.Montenovo M, Tatum RP, Figueredo E, et al. Does combined multichannel intraluminal esophageal impedance and manometry predict postoperative dysphagia after laparoscopic Nissen fundoplication? Dis Esophagus 2009; 22: 656–663 [DOI] [PubMed] [Google Scholar]
- 30.Tutuian R, Castell DO. Rumination documented by using combined multichannel intraluminal impedance and manometry. Clin Gastroenterol Hepatol 2004; 2: 340–343 [DOI] [PubMed] [Google Scholar]
- 31.Quiroga E, Cuenca-Abente F, Flum D, et al. Impaired esophageal function in morbidly obese patients with gastroesophageal reflux disease: Evaluation with multichannel intraluminal impedance. Surg Endosc 2006; 20: 739–743 [DOI] [PubMed] [Google Scholar]
- 32.Blonski W, Vela MF, Freeman J, et al. The effect of oral buspirone, pyridostigmine, and bethanechol on esophageal function evaluated with combined multichannel esophageal impedance-manometry in healthy volunteers. J Clin Gastroenterol 2009; 43: 253–260 [DOI] [PubMed] [Google Scholar]
- 33.Simrén M, Silny J, Holloway R, et al. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut 2003; 52: 784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A, Hila A, Tutuian R, et al. Bethanechol improves smooth muscle function in patients with severe ineffective esophageal motility. J Clin Gastroenterol 2007; 41: 366–370 [DOI] [PubMed] [Google Scholar]
- 35.Kim HS, Conklin JL, Park H. The effect of sildenafil on segmental oesophageal motility and gastro-oesophageal reflux. Aliment Pharmacol Ther 2006; 24: 1029–1036 [DOI] [PubMed] [Google Scholar]
- 36.Tutuian R, Mainie I, Allan R, et al. Effects of a 5-HT(4) receptor agonist on oesophageal function and gastro-oesophageal reflux: Studies using combined impedance-manometry and combined impedance-pH. Aliment Pharmacol Ther 2006; 24: 155–162 [DOI] [PubMed] [Google Scholar]
- 37.Cho YK, Choi MG, Oh SN, et al. Comparison of bolus transit patterns identified by esophageal impedance to barium esophagram in patients with dysphagia. Dis Esophagus 2012; 25: 17–25 [DOI] [PubMed] [Google Scholar]
- 38.Koya DL, Agrawal A, Freeman JE, et al. Impedance detected abnormal bolus transit in patients with normal esophageal manometry. Sensitive indicator of esophageal functional abnormality? Dis Esophagus 2008; 21: 563–569 [DOI] [PubMed] [Google Scholar]
- 39.Yigit T, Quiroga E, Oelschlager B. Multichannel intraluminal impedance for the assessment of post-fundoplication dysphagia. Dis Esophagus 2006; 19: 382–388 [DOI] [PubMed] [Google Scholar]
- 40.Dalmazo J, Aprile L, Dantas R. Esophageal contractions, bolus transit and perception of transit after swallows of liquid and solid boluses in normal subjects. Arq Gastroenterol 2012; 49: 250–254 [DOI] [PubMed] [Google Scholar]
- 41.Myers JC, Nguyen NQ, Jamieson GG, et al. Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol Motil 2012; 24: 812–e393 [DOI] [PubMed] [Google Scholar]
- 42.Imam H, Shay S, Ali A, et al. Bolus transit patterns in healthy subjects: A study using simultaneous impedance monitoring, videoesophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005; 288: G1000–G1006 [DOI] [PubMed] [Google Scholar]
- 43.Silny J, Knigge K, Fass J. Verification of the intraluminal multiple electrical impedance measurement for the recording of gastrointestinal motility. Neurogastroenterol Motil 1993; 5: 107–122 [Google Scholar]
- 44.Szczesniak MM, Rommel N, Dinning PG, et al. Intraluminal impedance detects failure of pharyngeal bolus clearance during swallowing: A validation study in adults with dysphagia. Neurogastroenterol Motil 2009; 21: 244–252 [DOI] [PubMed] [Google Scholar]
- 45.Szczesniak MM, Rommel N, Dinning PG, et al. Optimal criteria for detecting bolus passage across the pharyngo-oesophageal segment during the normal swallow using intraluminal impedance recording. Neurogastroenterol Motil 2008; 20: 440–447 [DOI] [PubMed] [Google Scholar]
- 46.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil 2004; 16: 533–542 [DOI] [PubMed] [Google Scholar]
- 47.Bulsiewicz WJ, Kahrilas PJ, Kwiatek MA, et al. Esophageal pressure topography criteria indicative of incomplete bolus clearance: A study using high-resolution impedance manometry. Am J Gastroenterol 2009; 104: 2721–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogte A, Bredenoord AJ, Oors J, et al. Relationship between esophageal contraction patterns and clearance of swallowed liquid and solid boluses in healthy controls and patients with dysphagia. Neurogastroenterol Motil 2012; 24: e364–e372 [DOI] [PubMed] [Google Scholar]
- 49.Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: The Chicago Classification. J Clin Gastroenterol 2008; 42: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omari TI, Dejaeger E, Van Beckevoort D, et al. A novel method for the nonradiological assessment of ineffective swallowing. Am J Gastroenterol 2011; 106: 1796–1802 [DOI] [PubMed] [Google Scholar]
- 51.Omari TI, Dejaeger E, van Beckevoort D, et al. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology 2011; 140: 1454–1463 [DOI] [PubMed] [Google Scholar]
- 52.Omari TI, Dejaeger E, Tack J, et al. Effect of bolus volume and viscosity on pharyngeal automated impedance manometry variables derived for broad dysphagia patients. Dysphagia 2013; 28: 146–152 [DOI] [PubMed] [Google Scholar]
- 53.Noll L, Rommel N, Davidson GP, et al. Pharyngeal flow interval: A novel impedance-based parameter correlating with aspiration. Neurogastroenterol Motil 2011; 23: 551–e206 [DOI] [PubMed] [Google Scholar]
- 54.Omari TI, Kritas S, Cock C, et al. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol Motil 2014; 26: 59–68 [DOI] [PubMed] [Google Scholar]
- 55.Omari T, Kritas S, Cock C. New insights into pharyngo-esophageal bolus transport revealed by pressure-impedance measurement. Neurogastroenterol Motil 2012; 24: e549–e556 [DOI] [PubMed] [Google Scholar]
- 56.Nguyen NQ, Holloway RH, Smout AJ, et al. Automated impedance-manometry analysis detects esophageal motor dysfunction in patients who have non-obstructive dysphagia with normal manometry. Neurogastroenterol Motil 2013; 25: 238–245, e164 [DOI] [PubMed] [Google Scholar]
- 57.Chen CL, Yi CH, Liu TT, et al. Characterization of esophageal pressure-flow abnormalities in patients with non-obstructive dysphagia and normal manometry findings. J Gastroenterol Hepatol 2013; 28: 946–953 [DOI] [PubMed] [Google Scholar]
- 58.Loots C, van Herwaarden MY, Benninga MA, et al. Gastroesophageal reflux, esophageal function, gastric emptying, and the relationship to dysphagia before and after antireflux surgery in children. J Pediatr 2013; 162: 566–573.e2 [DOI] [PubMed] [Google Scholar]
- 59.Omari TI, Wauters L, Rommel N, et al. Oesophageal pressure-flow metrics in relation to bolus volume, bolus consistency, and bolus perception. United Eur Gastroenterol J 2013; 1: 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rommel N, Van Oudenhove L, Tack J, et al. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol Motil 2014; 26: 636–645 [DOI] [PubMed] [Google Scholar]
- 61.Rohof WO, Myers JC, Estremera FA, et al. Inter- and intra-rater reproducibility of automated and integrated pressure-flow analysis of esophageal pressure-impedance recordings. Neurogastroenterol Motil 2014; 26: 168–175 [DOI] [PubMed] [Google Scholar]
- 62.Omari TI, Papathanasopoulos A, Dejaeger E, et al. Reproducibility and agreement of pharyngeal automated impedance manometry with videofluoroscopy. Clin Gastroenterol Hepatol 2011; 9: 862–867 [DOI] [PubMed] [Google Scholar]
- 63.Imam H, Marrero F, Shay S. Impedance nadir values correlate with barium bolus amount. Dis Esophagus 2012; 25: 600–607 [DOI] [PubMed] [Google Scholar]
- 64.Omari TI, Ferris L, Dejaeger E, et al. Upper esophageal sphincter impedance as a marker of sphincter opening diameter. Am J Physiol Gastrointest Liver Physiol 2012; 302: G909–G913 [DOI] [PubMed] [Google Scholar]
- 65.Kim HJ, Mittal RK, Patel N, et al. Esophageal distension during bolus transport: Can it be detected by intraluminal impedance recordings? Neurogastroenterol Motil 2014; 26: 1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kronecker KH, Meltzer SJ. Der Schluckmechanismus, seine Erregung und seine Hemmung. Arch Anat Phys 1883; 7: 328–362 [Google Scholar]
- 67.Buthpitiya AG, Stroud D, Russell CO. Pharyngeal pump and esophageal transit. Dig Dis Sci 1987; 32: 1244–1248 [DOI] [PubMed] [Google Scholar]
- 68.Gullung JL, Hill EG, Castell DO, et al. Oropharyngeal and esophageal swallowing impairments: Their association and the predictive value of the modified barium swallow impairment profile and combined multichannel intraluminal impedance-esophageal manometry. Ann Otol Rhinol Laryngol 2012; 121: 738–745 [DOI] [PubMed] [Google Scholar]
- 69.Omari TI, Dejaeger E, Tack J, et al. An impedance-manometry based method for non-radiological detection of pharyngeal postswallow residue. Neurogastroenterol Motil 2012; 24: e277–e284 [DOI] [PubMed] [Google Scholar]
- 70.Lin Z, Nicodème F, Lin CY, et al. Parameters for quantifying bolus retention with high-resolution impedance manometry. Neurogastroenterol Motil 2014; 26: 929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costa M, Wiklendt L, Arkwright JW, et al. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Front Syst Neurosci 2013; 7: 7–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dinning PG, Wiklendt L, Omari T, et al. Neural mechanisms of peristalsis in the isolated rabbit distal colon: A neuromechanical loop hypothesis. Front Neurosci 2014; 8: 75–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwiatek MA, Nicodème F, Pandolfino JE, et al. Pressure morphology of the relaxed lower esophageal sphincter: The formation and collapse of the phrenic ampulla. Am J Physiol Gastrointest Liver Physiol 2012; 302: G389–G396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwiatek MA, Pandolfino JE, Kahrilas PJ. 3D-high resolution manometry of the esophagogastric junction. Neurogastroenterol Motil 2011; 23: e461–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pandolfino JE, Ghosh SK, Lodhia N, et al. Utilizing intraluminal pressure gradients to predict esophageal clearance: A validation study. Am J Gastroenterol 2008; 103: 1898–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicodème F, Pandolfino JE, Lin Z, et al. Adding a radial dimension to the assessment of esophagogastric junction relaxation: Validation studies of the 3D-eSleeve. Am J Physiol Gastrointest Liver Physiol 2012; 303: G275–G280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Z, Imam H, Nicodème F, et al. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: A novel parameter for assessing esophageal bolus transit. Am J Physiol Gastrointest Liver Physiol 2014; 307: G158–G163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin Z, Pandolfino JE, Xiao Y, et al. Localizing the contractile deceleration point (CDP) in patients with abnormal esophageal pressure topography. Neurogastroenterol Motil 2012; 24: 972–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoikes N, Drapekin J, Kushnir V, et al. The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg Endosc 2012; 26: 3401–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abrahao L, Bhargava V, Babaei A, et al. Swallow induces a peristaltic wave of distension that marches in front of the peristaltic wave of contraction. Neurogastroenterol Motil 2011; 23: 201–207, e110 [DOI] [PubMed] [Google Scholar]
- 81.Wiklendt L, Costa M, Dinning PG. Inference of mechanical states of intestinal motor activity using hidden Markov models. BMC Physiol 2013; 13: 14–14 [DOI] [PMC free article] [PubMed] [Google Scholar]