Abstract
Purpose
Evaluating genetic susceptibility may clarify effects of known environmental factors and also identify individuals at high risk. We evaluated the association of four insulin-related pathway gene polymorphisms in insulin-like growth factor-1 (IGF-I) (CA)n repeat, insulin-like growth factor-2 (IGF-II) (rs680), insulin-like growth factor binding protein-3 (IGFBP-3) (rs2854744), and adiponectin (APM1 rs1501299) with colon cancer risk, as well as relationships with circulating IGF-I, IGF-II, IGFBP-3, and C-peptide in a population-based study.
Methods
Participants were African Americans (231cases, 306 controls) and Whites (297 cases, 530 controls). Consenting subjects provided blood specimens, and lifestyle/diet information. Genotyping for all genes except IGF-I was performed by the 5′-exonuclease (Taqman) assay. The IGF-I (CA)n repeat was assayed by PCR, and fragment analysis. Circulating proteins were measured by enzyme immunoassays. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression.
Results
The IGF-I (CA)19 repeat was higher in White controls (50%) than African American controls (31%). Whites homozygous for the IGF-I (CA)19 repeat had a nearly two fold increase in risk of colon cancer (OR=1.77; 95%CI=1.15–2.73), but not African Americans (OR= 0.73, 95%CI 0.50–1.51). We observed an inverse association between the IGF-II Apa1 A-variant and colon cancer risk (OR= 0.49, 95%CI 0.28–0.88) in Whites only. Carrying the IGFBP-3 variant alleles was associated with lower IGFBP-3 protein levels, a difference most pronounced in Whites (p- trend < 0.05).
Conclusions
These results support an association between insulin pathway-related genes and elevated colon cancer risk in Whites but not in African Americans.
Keywords: Insulin, IGF, Polymorphism, Colon cancer, African Americans
Introduction
For reasons that are as yet unclear, colorectal cancer incidence and mortality exhibit considerable race/ethnic variation, with the highest incidence in African Americans [1]. A combination of genetic, nutritional, and lifestyle factors may contribute to these differences [2]. However, most studies of colon cancer have been conducted in predominantly White populations that included few or no African Americans. Functional dysregulation of the insulin and the insulin-like growth factor (IGF) axis, which are major determinants of proliferation and apoptosis, have been hypothesized as a potential mechanism underlying colorectal carcinogenesis [2, 3]. We previously showed that elevated fasting insulin levels predicted increased risk of adenomas or colon cancer precursors [4]. Several epidemiologic studies including case-control [5, 6, 7] and cohort studies [8–13] have also evaluated the relationship between colon cancer, IGFs, and factors related to obesity and insulin resistance [14]. While the majority of these studies have reported modest positive associations [10–12, 15], other reports have found no associations [9, 11]. These inconsistent findings may be due, in part, to the complex and non-complementary relationship between components of the IGF axis, and genetic and environmental factors inherent in different ethnic/racial groups.
Investigating genetic factors related to the insulin–IGF pathway, including insulin-like growth factor-1 (IGF-I), insulin-like growth factor-2 (IGF-II), insulin-like growth factor binding protein-3 (IGFBP-3), adiponectin (APM1)- and their influence on their protein products circulating levels, in diverse cohorts, may help clarify previously reported inconsistent associations and also identify individuals at high risk. Insulin, IGF-I and IGF-II are growth factors that regulate cell proliferation, apoptosis, transformation and differentiation [16]. Insulin-like growth factor binding proteins (IGFBPs), primarily IGFBP-3 modulates the function of the IGFs [17]. IGF-I has a microsatellite (CA)n repeat polymorphism in the promoter region [18] shown to influence IGF-I production [19–25]. Similarly, a single nucleotide polymorphism (SNP) in IGFBP-3, -202A>C, is linked with low plasma IGFBP-3 levels [26, 27]. IGF-II is over-expressed in colon cancer [28, 29]. While loss of imprinting of the IGF-II gene may account for this observation [30, 31], an Apa1 polymorphism located in exon 9 of the IGF-II gene may also contribute [32], but has not been evaluated in relation to colon cancer. An equally attractive candidate gene is adiponectin (APM1), which encodes for an adipose secreted cytokine, adiponectin. This cytokine plays a key role in the regulation of glucose, metabolism of fatty acids [33], insulin resistance and inflammation. An intron 2 polymorphism (+ 276 G>T) in the APM1 gene, is associated with high circulating adiponectin levels and improved insulin sensitivity [34–36]. These insulin pathway-related genes and SNPs therein have been linked to colon cancer in smaller or Whites and Asian only studies [37–45].
Herein, we examined the association between polymorphisms in IGF-I (CA)n repeat, IGF-II (rs680), IGFBP-3 (rs2854744), and adiponectin (APM1 rs1501299) genes known to be either non-synonymous, or to influence protein production, and colon cancer risk. We hypothesized that genetic polymorphisms of these four SNPs may explain, at least in part, some of the racial/ethnic disparities in colon cancer incidence. In this study which included a large number of African Americans and Whites, we also examined the functional significance of these polymorphisms by examining their associations with circulating levels of IGF-I, IGF-II, IGFBP-3, and C-peptide among African American and white controls.
Methods
Study Population
Participants were enrollees of the North Carolina Colon Cancer Study – (NCCCS), a population based case-control study of participants from 33 contiguous counties of central North Carolina. Details of the study were previously published [35, 36]. Briefly, cases were ascertained through the North Carolina Central Cancer Registry rapid ascertainment system and consisted of persons with a first diagnosis of histologically confirmed invasive adenocarcinoma of the colon, between July 1996 and June 2000. All cases were between the ages of 40 and 85 at the time of diagnosis, residents of the 33-county study area, mentally competent to give informed consent and complete the interview, and possessed a North Carolina driver’s license or identification card. Controls younger than 65 years were randomly selected form the Division of Motor Vehicle records. The Health Care Financing Administration was the source for selecting controls aged 65 and older. They were frequency matched to cases by race, sex and 5-year age group. Completed interviews were obtained from 1,691 persons (634 cases and 1,048 controls), of whom 43% were African American. The overall study cooperation rate [interviewed/(interviewed + refused)] was 84% for cases and 63% for controls, whereas the response rate (interviewed/eligible) was 72% for cases and 61% for controls. Both cooperation and response rates were slightly higher for Whites than for African Americans, and reasons for the 12% difference in cooperation rate and response rate for cases included refusal to participate, MD (doctor) denied access to patient, untraceable or unable to contact as previously described [46, 47].
Data Collection
In-home-interviews were conducted by trained nurses to collect information about lifestyle and diet, as well as collect blood specimens under non-fasting conditions. A health history questionnaire was used to collect information on physical activity, anthropometric measurements (weight, height, waist, and hip), medical information, demographic information, smoking habits, family history, diabetes history, physical activity, NSAIDs use, and other factors that might relate to both colon cancer incidence and mortality. Detailed dietary information was collected using a modified version of the semi-quantitative food frequency questionnaire developed at the National Cancer Institute [48, 49]. The diet questionnaire was used to assess the frequencies and amounts of over 100 food items consumed during the year prior to diagnosis for cases with colon cancer or during the previous year for control subjects. A one-year period was chosen to provide a full cycle of seasons so that responses would be independent of the time of the year [50]. Nutrient intake was analyzed by the National Cancer Institute program, which incorporated the nutrient content of each food item, the consumption frequency, and a portion size based on age.
Blood Specimens
Blood samples were collected from consenting participants at the time of the interview. Cases were enrolled within 6 months of diagnosis. Blood was kept in coolers with cold packs until delivered to the laboratory for processing. Subjects who preferred to have the blood draw in their doctor’s office were provided a FedEx® specimen package for blood draw, and the blood specimens were shipped to the lab on cold packs. Blood was usually received and processed in the lab within 24 hours. Plasma samples were stored in aliquots at −80°C. The compliance rates for blood collection were 86% for cases and 83% for controls. Participants who gave blood were more likely to be male, White, and to have never smoked compared with those who did not give blood. These groups did not differ according to dietary intake or other risk factors including stage at diagnosis in cases. Odds ratios for colon cancer and dietary and other risk factors did not differ between those who gave blood and those who did not (data not shown).
DNA extraction
Genomic DNA was extracted from peripheral blood leukocytes using the PureGene DNA isolation kit (Gentra Systems, Inc., Minneapolis, MN).
Genotyping
Genotyping was performed using the ABI 7700 Sequence Detection System 5′ exonuclease (Taqman™) assay. IGF-II, IGFBP-3 and APM1 (Adiponectin) primers and probes were designed using Primer Express™ oligonucleotide design software (Applied Biosystems, Forster City, CA). For IGF-II the probe was 5′-AAAAGAAGGGCCCCAGA -3′, the F-primer was 5′-GAGTCCCTGAACCAGCAAAGAG -3′, and the R-primer was 5′-AAATTCCCGTGAGAAGGGAGAT-3′. For IGFBP-3, the probe, the F- and R- primers were: 5′-CTCGTGCTCACGCC -3′; 5′-ACACCTTGGTTCTTGTAGACGACAA -3′, and 5′-GGCGTGCAGCTCGAGACT -3′, respectively. The assay design and conditions were based on the allelic discrimination protocol from Applied Biosystems. Similar PCR reaction conditions were used for these genotype assays. Briefly, reactions were performed in a final volume of 15μl containing 30 ng of genomic DNA, 900nM of each primer, 100nM of each probe, and 0.7X Taqman Universal PCR Master Mix. Amplifications were performed on Perkin Elmer GenAmp® 9700 thermocyclers using the 9600 mode under the following conditions: 50°C for 2 minutes (AmpErase® UNG), 95°C for 10 minutes followed by 35 cycles of 95°C for 15 seconds, and 64°C for 1 minute.
For Adiponectin genotyping, PCR primers and probes were commercially designed by Assays by Design (Applied Biosystems), and as such the probe and primer sequences are proprietary. The reaction components were as follows: 2X Taqman Universal PCR master mix, 20X Applied Biosystems primer and probe mix and 15 ng of genomic DNA. The reaction conditions were as follows: 50°C for 2 minutes, 1 cycle of 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute.
For all Taqman genotyping assays, plates were read on the ABI 7700 using the allele discrimination protocol. Six of each non-template (water) controls and amplification/genotype controls were included in each run. Positive controls included DNA samples from the Coriell tissue repository that have been previously sequenced by SNP500 cancer [51]. All samples were successfully amplified and genotyped. For each gene, assays were also repeated on 10% of randomly selected samples, and the results were 100% concordant.
IGF-I (CA)19 genotyping methods
Analysis of the cytosine-adenine (CA)n repeats of the IGF-I gene, located 1 kb upstream from the transcription start site was performed using PCR and fragment analysis. The PCR reactions performed in 50μl volumes, consisted of 10 ng of genomic DNA using 125 nM of each primer (fluorescently labeled forward primer, 5′ 6-FAM-GCTAGCCAGCTGGTGTTATT-3 and reverse, 5′-ACCACTCTGGGAGAAGGGTA-3), 100 μM dNTPs, 1.5 mM MgCl2, and 1.5 units of AmpliTaq Gold polymerase (ABI Applied Biosystems), and standard PCR buffer. Amplification cycles included one cycle of 15 minutes at 95°C, 35 cycles consisting of 30 seconds at 95°C (denaturation), 30 seconds at 64°C (annealing), and 1 minute at 72°C (elongation), and a final elongation step at 72°C for 7 minutes. Amplified PCR products were purified over QIAquick purification columns (Qiagen), followed by a 1:100 dilution. The diluted product (10μl) was mixed with loading buffer (80% formamide, 5mM EDTA, 50 ug/uL Blue Dextran) and ROX GeneScan-350 size standard (ABI) followed by denaturing for 5 minutes at 95°C and capillary electrophoresis on the ABI-3600 genetic analyzer. Fragment sizing was determined by Genescan analyses software (ABI Applied Biosystems). The fragments ranged in size from 174 to 202 base pairs, depending on the number of CA repeats.
DNA from representative homozygote individuals for the (CA)18, (CA)19, and (CA)20 genotypes were sequenced to confirm the number of CA repeats. Quality control procedures were as follows, blinding of lab personnel to subjects’ case-control status, inclusion of positive and negative controls in each assay run, and review of genotype results by a second reviewer (TOK). In addition, 10% of samples were repeated blindly to validate the genotyping procedures. The concordance for the blinded repeat samples was 100%.
Enzyme Linked Immunosorbent Assays
Plasma levels of IGF-I, IGF-II, IGFBP-3, and C-peptide were measured by ELISA using commercially available kits (DSL Inc. Webster, Texas). IGF-I and IGF-II were measured after acid-ethanol extraction to remove IGFBPs. These analytes were measured in duplicate on stored plasma samples and laboratory personnel were blinded to the case or control status of samples. Intra-assay coefficients of variation were 6.5% for IGF-I, 1.5% for IGF-II, 4.7% for IGFBP-3, and 5.7% for C-peptide. The inter-assay coefficient of variation was less than 12% for all of the analytes measured. Previous epidemiologic studies have reported good reproducibility with these commercial kits [10, 52].
Data analysis
For cases and controls, descriptive statistics were generated for age (<65 years/>=65 years), race (White/black), gender (male/female), family history (yes/no for at least one first-degree relative with colon cancer), diabetes history (yes/no), BMI one year ago or one year prior to diagnosis for cases (categorized into normal, overweight and obese), NSAID usage (use >= 15 NSAIDs/month in past 5 years), alcohol use, (yes/no), and smoking status (never, former, current). Also, mean values as well as number above and below the median value (as determined by controls) was found for waist-hip ratio, physical activity as measured in MET-minutes per day, total daily calories, percentage of calories from fat, and total daily calcium intake. P-values were generated to compare cases and controls.
Allele frequencies were calculated as the number of copies of a particular allele divided by the total number of chromosomes (2N=two times the number of persons in the study). Genotype frequencies were calculated as the number of participants having each genotype divided by the total number of participants. Exact 95% confidence intervals for allele and genotype frequencies were computed by the method outlined by Collett [53]. Distributions for cases and controls were compared via chi-square tests. Observed genotype frequencies were compared to expected genotype frequencies, calculated on the basis of observed allelic frequencies, assuming Hardy-Weinberg equilibrium. Departure from Hardy-Weinberg equilibrium was tested among control groups using a goodness-of-fit chi-square test.
Unconditional logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between carrying the variant allele and risk of colon cancer. We compared carriers of at least one of the minor alleles (at risk genotype) to individuals homozygous for the common allele (referent genotype). All statistical models were adjusted for colon cancer risk confounders in the population, including age at reference date, sex and race; race was excluded from race-stratified models. Factors previously reported to be associated with colon cancer including body mass index (BMI = kg/m2) and NSAID use were explored for effect modification using stratified analyses. These factors were then evaluated for confounding (together with family history, alcohol use, fat calories, physical activity, caloric intake, diabetes history, age, race, and current and former cigarette smoking) comparing logistic regression models with and without the variables of interest. Regression models were used to evaluate the correlation between genotype and circulating protein concentrations, adjusting for potential confounders. SAS version 9.1 (SAS, Cary, NC, USA) was used for all statistical analyses.
Results
Descriptive characteristics of cases and controls are summarized in Table 1. Briefly, 48% of cases were less than 65 years of age compared to 38% of controls (p=0.0001); 43% of the 553 cases compared to 38% of 875 controls were African American (p=0.001); and women comprised 48% of cases and 48% of controls. Cases did not differ considerably from controls with respect to diabetes, BMI recalled a year prior to the interview, waist/hip ratio, physical activity, and alcohol use. However, cases were more likely than controls to report a family history of colon cancer (21% vs 9%, respectively), higher average calorie consumption per day, and greater proportion of calories derived from fat. Sixty two percent of cases were current or former cigarette smokers compared to 59% of controls (p=0.03), and cases were less likely to have used >=15 NSAIDS/month in the past five years (p=0.05).
Table 1.
Descriptive characteristics of study participants
| Whites | African Americans | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic1 | Cases (n=312) | Controls (n=545) | p-value | Cases (n=240) | Controls (n=328) | p-value |
| Age | ||||||
| < 65 | 133 (43) | 198 (36) | 0.0001 | 131 (55) | 134 (41) | 0.0001 |
| >= 65 | 179 (57) | 347 (64) | 109 (45) | 194 (59) | ||
| Race | ||||||
| Caucasian | 313 (57) | 546 (62) | 0.001 | 313 (57) | 546 (62) | 0.001 |
| African American | 240 (43) | 329 (38) | 240 (43) | 329 (38) | ||
| Gender | ||||||
| Female | 140 (45) | 241 (44) | 0.25 | 123 (51) | 176 (54) | 0.06 |
| Male | 172 (55) | 304 (56) | 117 (49) | 152 (46) | ||
| Family History of colon cancer | ||||||
| No | 242 (78) | 487 (90) | 0.0001 | 195 (81) | 300 (92) | 0.0001 |
| Yes | 68 (22) | 52 (10) | 45 (19) | 27 (8) | ||
| Diabetes | ||||||
| No | 256 (82) | 475 (87) | 0.03 | 175 (73) | 239 (73) | 0.70 |
| Yes | 55 (18) | 69 (13) | 64 (27) | 89 (27) | ||
| BMI 1 year ago (categorical) | ||||||
| Normal (18–24.9) | 84 (28) | 161 (30) | 0.56 | 34 (15) | 62 (20) | 0.13 |
| Overweight (25–29.9) | 127 (42) | 227 (43) | 97 (42) | 114 (37) | ||
| Obese (>=30) | 92 (30) | 140 (27) | 98 (43) | 130 (43) | ||
| Physical Activity (MET-minutes per day) (mean (SD)) | 2,240 (534) | 2,207 (509) | 0.55 | 2,259 (611) | 2,142 (522) | 0.22 |
| Calories (kcal per day) (mean (SD)) | 1,987 (721) | 1,804 (602) | 0.43 | 1,973 (857) | 1,733 (689) | 0.002 |
| Smoking | ||||||
| Never | 103 (33) | 217 (40) | 0.01 | 106 (45) | 143 (44) | 0.57 |
| Former | 266 (54) | 244 (45) | 81 (34) | 110 (44) | ||
| Current | 41 (13) | 83 (15) | 50 (21) | 75 (23) | ||
| Used >= 15 NSAIDs per month in past 5 years | ||||||
| Yes | 97 (31) | 194 (36) | 0.32 | 48 (20) | 96 (29) | 0.06 |
| No | 215 (69) | 351 (64) | 191 (80) | 232 (71) | ||
Categorical variables are given as n (%). Continuous variables are given as mean and standard deviation (se).
The distribution of IGF-I (CA)19 polymorphism for African American and White cases and controls is shown in Figure 1. IGF-I (CA)n polymorphic repeats ranged from n=11 (fragment size 174 bp) to n=24 repeats (fragment size 202 bp) and the most common repeat was n =19 (fragment size 192 bp) which was designated the referent allele. Overall, the IGF-I (CA)19 allele was the most common, present in 46% of cases and 43% of controls. Among African Americans, 30% of cases and 31% of controls carried the IGF-I (CA)19 allele while the next most frequent allele, (CA)18, was found in 25% of cases and 26% of controls. Among Whites, 58% of cases and 50% of controls carried the (CA)19 allele while the next most frequent allele, (CA)20, had a distribution of 21% in cases and 25% in controls. Although, the IGF-I (CA)19 allele distribution differed by race with higher frequencies observed in Whites than African Americans, the distributions were similar to those published in the literature for Whites [25] and African Americans [54–57].
Fig. 1. Distribution of IGF-I alleles among African Americans and Whites.
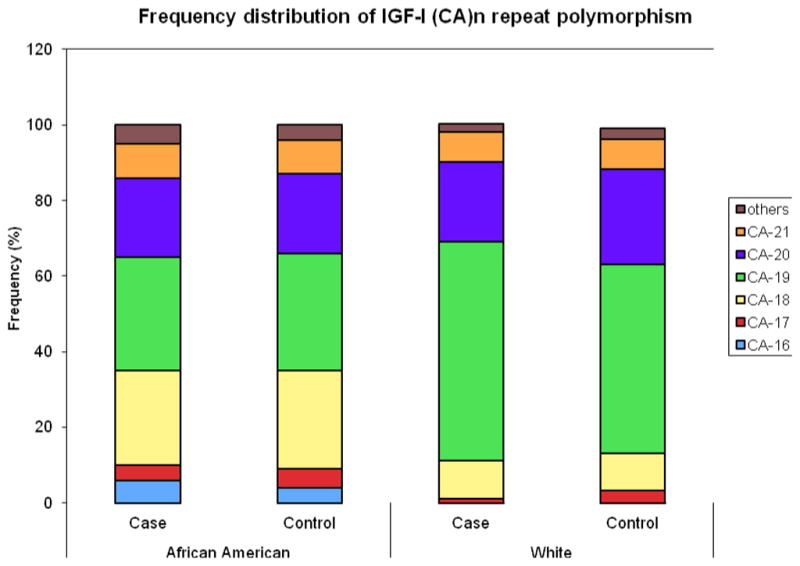
IGF-I (CA)n polymorphic repeats ranged from n=11 (fragment size 174 bp) to n=24 repeats (fragment size 202 bp) and the most common repeat was n=19 (fragment size 192 bp). IGF-I (CA)19 is the most frequent allele among African American and Whites cases and controls
Sex, age, calories, NSAIDS and cigarette smoking adjusted ORs and 95%CI for associations between IGF-I, IGFBP-3, IGF-II and AMP1 genetic variants and colon cancer risk in African American and Whites, are shown in Table 2. Whites homozygous for the IGF-I (CA)19 repeat polymorphism had a nearly 2-fold, dose-dependent increased risk of colon cancer (OR=1.77; 95%CI=1.15–2.73). We also found a dose-dependent inverse association between homozygous carriers of the IGF-II Apa1 A-variant and colon cancer risk in Whites (OR= 0.49, 95%CI 0.28–0.88). These associations were not apparent in African Americans. We also found little evidence for associations between carrying the IGFBP-3 or the APM1 variants and colon cancer risk in Whites and African Americans. None of the genotype distributions among controls deviated from Hardy Weinberg Equilibrium (IGF-II Apa1 p=0.99, IGFBP-3 p=0.99, and AMP1 p=0.72).
Table 2.
ORs and 95%CI1 for the associations between IGF-I, IGFBP-3, IGF-II and APMI genetic variants and colon cancer risk in African American and White carriers
| African American | Whites | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Gene/genotype | Genotype Frequency | OR1 (95%CI) | Genotype Frequency | OR1 (95%CI) | ||
| Case n(%) | Control n(%) | Case n(%) | Control n(%) | |||
| IGF-II(Apa 1) | ||||||
| GG | 203 (85) | 284 (87) | ref. | 172 (55) | 259 (48) | ref. |
| GA | 36 (15) | 40 (12) | 1.14 (0.68–1.93) | 120 (38) | 231 (43) | 0.82 (0.60–1.11) |
| AA | 1 (0.4) | 1 (0.3) | 1.88 (0.11–30.48) | 20 (6) | 51 (9) | 0.49 (0.28–0.88) |
| AA+GA | 37 (15) | 41 (13) | 1.16 (0.69–1.4) | 140 (45) | 283 (52) | 0.75 (0.56–1.01) |
|
| ||||||
| IGF-I (CA)19 | ||||||
| Non/Non | 107 (48) | 136 (46) | ref. | 54 (19) | 118 (23) | ref. |
| (CA)19/Non | 99 (44) | 136 (46) | 0.87 (0.59–1.27) | 33 (46) | 269 (53) | 1.09 (0.73–1.63) |
| (CA)19/(CA)19 | 18 (8) | 24 (8) | 0.73 (0.50–1.51) | 101 (35) | 124 (24) | 1.77 (1.15–2.73) |
| (CA)19/Non and (CA)19/(CA)19 | 117 (52) | 160 (54) | 0.84 (0.58–1.22) | 114 (81) | 225 (77) | 1.31 (0.90–1.91) |
|
| ||||||
| IGFBP-3 | ||||||
| AA | 83 (35) | 106 (33) | ref. | 75 (24) | 128 (24) | ref. |
| AC | 111 (46) | 153 (47) | 0.93 (0.62–1.38) | 141 (45) | 272 (50) | 0.87 (0.61–1.25) |
| CC | 46 (19) | 66 (20) | 0.97 (0.59–1.60) | 95 (31) | 141 (26) | 1.13 (0.76–1.69) |
| CC+AC | 157 (65) | 219 (67) | 0.94 (0.65–1.37) | 237 (76) | 413 (76) | 0.96 (0.68–1.35) |
|
| ||||||
| APMI | ||||||
| GG | 78 (41) | 116 (42) | ref. | 122 (48) | 223 (48) | ref. |
| GT | 94 (49) | 119 (43) | 1.06 (0.69–1.62) | 106 (42) | 204 (44) | 0.92 (0.66–1.29) |
| TT | 19 (10) | 41 (15) | 0.63 (0.32–1.24) | 27 (11) | 36 (8) | 1.49 (0.85–2.61) |
| GT+TT | 113 (59) | 160 (58) | 0.95 (0.63–1.43) | 133 (52) | 240 (52) | 1.00 (0.73–1.38) |
Adjusted for age, sex, calories, NSAIDs and smoking.
Table 3 shows plasma levels of IGF-II, IGF-I, IGFBP-3, and C-peptide among controls carriers of IGF axis genes polymorphisms. Overall, circulating levels of IGF-I, IGF-II, IGFBP-3 and C-peptide were similar in Whites and African Americans. Within each ethnic/race group, plasma concentrations of IGF-I and IGF-II also did not vary significantly by genotype. We then analyzed C-peptide concentrations, a stable surrogate for measuring insulin levels, in carriers of the AMP1 SNP, since polymorphisms of the adiponectin gene are associated with insulin resistance and type II diabetes [58, 59], known confounders of colon cancer risk. Compared to non-carriers, we observed lower C-peptide concentrations among carriers of the AMP1 genotype in African Americans (p=0.13) and Whites (p=0.36) although not statistically significant. In Whites, non-carriers of the IGFBP-3 variant had significantly higher plasma levels of IGFBP-3 (3543 ng/ml, SE=110) than either homozygous carriers (3188 ng/ml, SD=104, p=0.03) or heterozygous and homozygous carries (3239 ng/ml, SE=80) (p=0.008), an association not found in African American. The ratio of IGF-I/IGFBP-3 levels showed higher concentrations in White and not in African American carriers of the IGFBP-3 variant, however not in carriers of the IGF-I (CA)19 repeat.
Table 3.
Mean plasma levels1 of IGF-I, IGF-II, IGFBP-3, and C-peptide among controls carriers of IGF-axis gene polymorphisms.
| African Americans | Whites | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Gene/genotype | N | Plasma level | p-value | N | Plasma level | p-value |
| IGF-II ng/ml (mean, se) | IGF-II ng/ml (mean, se) | |||||
| IGF-II | ||||||
| GG | 266 | 524 (12) | 254 | 592 (14) | ||
| GA | 36 | 497 (29) | 221 | 595 (15) | ||
| AA | 1 | 567 (167) | 0.64 | 51 | 569 (30) | 0.72 |
| AA +GA | 37 | 499 (28) | 0.40 | 272 | 591 (14) | 0.94 |
|
| ||||||
| IGF-I ((CA)19 repeat) | IGF-I ng/ml (mean, se) | IGF-I ng/ml (mean, se) | ||||
| Non/Non | 136 | 123 (6) | 118 | 120 (6) | ||
| (CA)19/Non | 136 | 126 (6) | 269 | 123 (4) | ||
| (CA)19/(CA)19 | 24 | 113 (13) | 0.62 | 123 | 118 (5) | 0.72 |
| (CA)19/Non and | 160 | 125 (6) | 0.80 | 392 | 121 (3) | 0.88 |
|
| ||||||
| IGFBP-3 ng/ml (mean, se) | IGFBP-3 ng/ml (mean, se) | |||||
| IGFBP-3 | ||||||
| AA | 101 | 3144 (117) | 123 | 3543 (110) | ||
| AC | 144 | 2921 (101) | 264 | 3239 (80) | ||
| CC | 57 | 3034 (160) | 0.31 | 138 | 3188 (104) | 0.03 |
| CC+AC | 201 | 2950 (90) | 0.17 | 402 | 3221 (67) | 0.008 |
|
| ||||||
| C-peptide ng/ml (mean, se) | C-peptide ng/ml (mean, se) | |||||
| APMI | ||||||
| GG | 115 | 2.54 (0.18) | 221 | 2.76 (0.15) | ||
| GT | 119 | 2.15 (0.17) | 204 | 2.57 (0.17) | ||
| TT | 39 | 2.37 (0.29) | 0.25 | 36 | 2.67 (0.35) | 0.64 |
| GT+TT | 158 | 2.20 (0.16) | 0.13 | 240 | 2.59 (0.16) | 0.36 |
|
| ||||||
| IGF-I ((CA)19 repeat) | IGF-I ng/ml/IGFBP-3 ng/ml (mean, se) | IGF-I ng/ml/IGFBP-3 ng/ml (mean, se) | ||||
| Non/Non | 136 | 0.042 (0.002) | 117 | 0.038 (0.001) | ||
| (CA)19/Non | 136 | 0.042 (0.002) | 269 | 0.038 (0.001) | ||
| (CA)19/(CA)19 | 24 | 0.040 (0.004) | 0.91 | 123 | 0.036 (0.001) | 0.76 |
| (CA)19/Non and | 160 | 0.041 (0.002) | 0.94 | 392 | 0.037 (0.001) | 0.55 |
|
| ||||||
| IGF-I ng/ml/IGFBP-3 ng/ml (mean, se) | IGF-I ng/ml/IGFBP-3 ng/ml (mean, se) | |||||
| IGFBP-3 | ||||||
| AA | 101 | 0.040 (0.002) | 123 | 0.034 (0.001) | ||
| AC | 144 | 0.040 (0.002) | 264 | 0.037 (0.001) | ||
| CC | 57 | 0.045 (0.002) | 0.14 | 138 | 0.039 (0.001) | 0.02 |
| CC+AC | 201 | 0.042 (0.001) | 0.35 | 402 | 0.038 (0.001) | 0.01 |
Adjusted for age, sex, calories, NSAIDs and smoking.
Discussion
In this population-based case control study, we evaluated the associations of four SNPs in IGF-I, IGF-II, IGFBP-3 and adiponectin, and colon cancer risk in African Americans and Whites. We also examined associations between these genotypes and their protein products among population controls. After adjusting for known confounders, we found that carrying the IGF1 (CA)19 repeat polymorphism was associated with an increase in colon cancer risk while carrying the IGF-II Apa1A variant was associated with lower risk. This association was limited to Whites. However, we found no evidence for an association between carrying these variants and circulating protein products, IGF1 and IGF2. We also found that carrying the IGFBP-3 and AMP1 variants was not associated with risk of colon cancer in either ethnic group. However, among otherwise healthy individuals, carrying the IGFBP-3 polymorphism was associated with lower IGFBP-3 levels in Whites, and a trend for lower IGFBP-3 levels in African Americans. IGF-I/IGFBP-3 ratio concentrations were significantly higher in White carriers of the IGFBP-3 SNP, but no differences were observed in White carriers of the IGF1 (CA)19 repeat variant nor in African American carriers of these SNPs, indicating that the IGFBP-3 SNP may influence lower expression of IGFBP-3 and perhaps colon cancer risk in Whites.
Although twin studies suggest that serum levels of IGF-I, IGF-II and IGFBP-3 may be influenced by a combination of genetic and environmental factors [21, 60–62], few epidemiologic studies have considered the impact of the genetic component on plasma levels when examining associations with colon cancer. To our knowledge, this is the first population-based study that includes a significantly large number of African Americans, to report on four insulin-pathway related genes polymorphisms and colon cancer risk and simultaneous evaluation of their influence on circulating protein plasma levels among controls. The frequencies for IGF-I (CA)19 repeat polymorphism in African Americans and Whites were within the ranges previously reported by others [25, 54, 55, 57] and HapMap [63]. Our findings that homozygosity for the IGF-I (CA)19 repeat polymorphism was associated with increased risk of colon cancer among Whites are consistent with the majority of studies that have evaluated the IGF-I(CA)19 repeat polymorphism and colon cancer [64] although the association with protein concentrations differ. While some studies reported correlation with reduced IGF-I levels [23, 25], others found that the IGF-I (CA)19 repeat did not predict plasma levels [27, 65]. However, these findings are consistent with those of our previous reports where the effects of the IGF-I (CA)19 polymorphism on circulating concentrations of IGF-I varied considerably by race/ethnicity [66], and associated with prostate cancer risk [67]. A recent study in a Chinese population, further analyzed the potential causes of these ethnic differences and found that the IGF-I (CA)19 repeat by itself is not the primary regulatory element of IGF-I expression [68]. The null results among African Americans in the present study, could be due to their lower frequency of the IGF-I (CA)19, repeat, or to recently identified factors, including other genetic variants in the IGF-I regulatory region which may be contributing to the regulation of IGF-I expression. In addition, the high degree of linkage disequilibrium in the IGF-I promoter region cannot be fully measured by association studies [68]. Furthermore, IGF-I/IGFBP-3 ratio levels did not vary in African American and White carriers of the IGF-I (CA)19, repeat SNP, however, IGF-I/IGFBP-3 ratio levels were higher in White carriers of the IGFBP-3 SNP, suggesting that lower levels of IGFBP-3 may be influencing higher free IGF-I in circulation, a combination of factors that has been previously shown to be independently associated with an increased risk of colorectal adenoma and cancer in women [15].
Neither African American nor White carriers of the IGFBP-3 polymorphism had increased colon cancer risk. However, among otherwise healthy individuals, carriers in both populations had significantly lower IGFBP-3 plasma levels, compared to non-carriers, although a dose-dependent trend was not apparent. Lower circulating IGFBP-3 levels have been associated with poor prognosis among colorectal cancer patients receiving chemotherapy [69, 70] while higher IGFBP-3 plasma levels associated with decreased colon cancer risk [8, 13]. A human colorectal carcinogenesis genome-wide study recently identified missense mutations in the IGFBP-3 gene, which could be modulating IGFBP-3 transcripts and subsequent protein expression [69]. These mutations could be in linkage disequilibrium with the IGFBP-3 polymorphism and thus explain the association between lower IGFBP-3 protein levels and CC risk in controls, however the IGFBP-3 polymorphism by itself was not associated with CC risk.
The role of the exon 9 Apa I polymorphism of the IGF-II gene in colon cancer is unclear, although some studies suggest that circulating levels of IGF-II and local tissue expression may be influenced by loss of imprinting [71]. In this study, the IGF-II Apa1 AA variant genotype was inversely associated with colon cancer risk among men and women particularly among Whites (data not shown). Those carrying the IGF-II Apa1 A-variant had somewhat lower circulating levels of this potent growth factor compared to those without the variant. This contrasts with the findings by Ma et. al. [10] who reported no association between IGF-II levels and colorectal cancer risk in a case-control study. It is possible that the influence of the A-variant on protein levels is less than what would be achieved by IGF-II relaxation of imprint controls, since IGF-II is an imprinted gene.
Obesity, central adiposity, and increased energy intake are associated with elevated risk of colon cancer [3, 72, 73] via their effects on insulin levels, insulin resistance [74] and inflammation. Adiponectin, an adipose secreted cytokine, is responsible for enhanced insulin sensitization [75] and has been associated with known colon cancer risk factors namely obesity, type 2 diabetes, and inflammation [5, 76–80]. Plasma adiponectin levels have been evaluated relative to colon cancer with contradictory results. Wei et al. [81] observed that low plasma adiponectin was associated with increased risk of colorectal cancer among men, while another study observed no association between plasma adiponectin and colorectal cancer [82]. We reasoned that genetic variation in adiponectin may contribute to these conflicting reports. Several adiponectin variants have been shown to affect adiponectin levels and have been associated with insulin resistance, obesity, and type 2 diabetes [83]. However, few studies have evaluated adiponectin polymorphisms in relation to colon cancer. We examined a polymorphism in the adiponectin gene (276G>T) and observed a lower colon cancer risk in African American carriers of this variant. We also found a decrease in C-peptide for the T-allele in both African Americans and Whites. The T allele is associated with elevated levels of adiponectin [83, 84] and improved insulin sensitivity [78], therefore our findings of reduced plasma C-peptide among controls with the T-allele supports previous observations. Recent reports showed that lower levels of prediagnosis plasma C-peptide among patients with surgically resected colorectal cancer associated with decreased mortality [85], whereas elevated concentrations of C-peptide may predict the risk of developing colorectal cancer [86], however genetic factors were not analyzed in these studies. Although our findings should be interpreted with caution since we measured non-fasting C-peptide concentrations, mean levels (0.84 (0.72–0.96) pmol/mL and 0.92 (0.82–1.01) pmol/mL) in African American and White controls, respectively) were strikingly similar to mean and fifth quintile levels reported by others [48] under the same non-fasting conditions (mean=0.70 (0.59–0.82) pmol/mL) (Q5=0.97 (0.74–2.02) pmol/mL). Our findings, even though not statistically significant showed that African American and White APMI variant carriers had lower levels of circulating C-peptide (0.73 (0.62–0.83) and 0.86 (0.75–0.96) pmol/mL, respectively). Hara et al. [84] found that among subjects with high BMI, the GG genotype was related to increased insulin resistance and low levels of adiponectin.
Although this is not a genome-wide study, and we cannot exclude the influence of other genes or other SNPs in these pathways, only one measure of proteins was made, yet there may be considerable variation in plasma levels over time, and C-peptide levels were measured under non-fasting conditions. However, our results relating protein differences to colon cancer risk are compatible with observations from cohort [9, 10, 86, 87] and other case-control studies [88]. Our findings of racial differences in both the association of genotypes on colon cancer risk and effects on circulating protein levels are novel and contribute to the existing literature on insulin-IGF axis and insulin resistance-related factors strong associations with colon cancer. In a previous study of 70 African Americans and Caucasians, we found that the effect of genetic and lifestyle factors such as cigarette smoking and IGF-I CA repeats influenced IGF1 and IGFBP-3 plasma levels in one race group only [65].
In summary, this study provides evidence for associations between heritable insulin–IGF axis factors and colon cancer risk -a risk most pronounced in Whites. The association between the insulin-IGF axis genetic variants and circulating protein products will require replication in larger studies where ancestral markers are used to define race/ethnicity.
Acknowledgments
This project was supported in part by grants from the National Institutes of Health K01 CA93654, P30 DK 034987 and R01 CA 66635, P50 CA 106991 and the Howard Hughes Medical Institute (HHMI) support for the UNC Program in Minority access to Biomedical Science (PMABs).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 4.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2076–2081. doi: 10.1158/1055-9965.EPI-05-0239. [DOI] [PubMed] [Google Scholar]
- 5.Le Marchand L, Kolonel LN, Henderson BE, Wilkens LR. Association of an exon 1 polymorphism in the IGFBP3 gene with circulating IGFBP-3 levels and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14:1319–1321. doi: 10.1158/1055-9965.EPI-04-0847. [DOI] [PubMed] [Google Scholar]
- 6.Slattery ML, Murtaugh M, Caan B, Ma KN, Neuhausen S, Samowitz W. Energy balance, insulin-related genes and risk of colon and rectal cancer. Int J Cancer. 2005;115:148–154. doi: 10.1002/ijc.20843. [DOI] [PubMed] [Google Scholar]
- 7.Wernli KJ, Newcomb PA, Wang Y, Makar KW, Shadman M, Chia VM, Burnett-Hartman A, Wurscher MA, Zheng Y, Mandelson MT. Body size, IGF and growth hormone polymorphisms, and colorectal adenomas and hyperplastic polyps. Growth Horm IGF Res. 2010;20:305–9. doi: 10.1016/j.ghir.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannucci E, Pollak MN, Platz EA, Willet WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–349. [PubMed] [Google Scholar]
- 9.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 11.Nomura AM, Stemmermann GN, Lee J, Pollak MN. Serum insulin-like growth factor I and subsequent risk of colorectal cancer among Japanese-American men. Am J Epidemiol. 2003;158:424–431. doi: 10.1093/aje/kwg176. [DOI] [PubMed] [Google Scholar]
- 12.Palmqvist R, Hallmans G, Rinaldi S, Biessy C, Stenling R, Riboli E, Kaaks R. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–646. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst-Hensch NM, Yuan JM, Stanczyk FZ, Gao YT, Ross RK, Yu MC. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai men. Br J Cancer. 2001;85:1695–1699. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006;17:145–56. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Pollak M, Platz EA, Willet WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses’ Health Study. Growth Horm IGF Res. 2000;10(Suppl A):S30–1. doi: 10.1016/s1096-6374(00)90014-5. [DOI] [PubMed] [Google Scholar]
- 16.Sridhar SS. Insulin-Insulin-like growth factor axis and colon cancer. J Clin Oncol. 2009;27:165–167. doi: 10.1200/JCO.2008.19.8937. [DOI] [PubMed] [Google Scholar]
- 17.McElhom AR, McKnight A-J, Patterson CC, Johnston BT, Hardie LJ, Murray LJ The Finbar Group. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology. 2010;139:204–212. doi: 10.1053/j.gastro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986;261:4828–4832. [PubMed] [Google Scholar]
- 19.DeLellis K, Ingles S, Kolonel L, McKean-Codwin R, henderson B, Stanczyk F, Probst-Hensch NM. IGF1 genotype, mean plasma level and breast cancer risk in the Hawaii/Los Angeles multiethnic cohort. Br J Cancer. 2003;88:277–282. doi: 10.1038/sj.bjc.6600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frayling TM, Hattersley AT, McCarthy A, Holly J, Mitchell SM, Gloyn AL, Owen K, Davies D, Smith GD, Ben-Shlomo Y. A putative functional polymorphism in the IGF-I gene: association studies with type 2 diabetes, adult height, glucose tolerance, and fetal growth in U.K. populations. Diabetes. 2002;51:2313–2316. doi: 10.2337/diabetes.51.7.2313. [DOI] [PubMed] [Google Scholar]
- 21.Harrela M, Koistinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, Toivanen L, Koskenuvo M, Leinonen P, Koistinen R, Seppala M. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Missmer SA, Haiman CA, Hunter DJ, Willet WC, Colditz GA, Speizer FE, Pollak MN, Hankinson SE. A sequence repeat in the insulin-like growth factor-1 gene and risk of breast cancer. Int J Cancer. 2002;100:332–336. doi: 10.1002/ijc.10473. [DOI] [PubMed] [Google Scholar]
- 23.Rietveld I, Janssen JA, Hofman A, Pols HA, van Duijn CM, Lamberts SW. A polymorphism in the IGF-I gene influences the age-related decline in circulating total IGF-I levels. Eur J Endocrinol. 2003;148:171–175. doi: 10.1530/eje.0.1480171. [DOI] [PubMed] [Google Scholar]
- 24.Rosen CJ, Kurland ES, Vereault D, Adler RA, Rackoff PJ, Craig WY, Witte S, Rogers J, Bilezikian JP. Association between serum insulin growth factor-I (IGF-I) and a simple sequence repeat in IGF-I gene: implications for genetic studies of bone mineral density. J Clin Endocrinol Metab. 1998;83:2286–2290. doi: 10.1210/jcem.83.7.4964. [DOI] [PubMed] [Google Scholar]
- 25.Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, Lamberts SW, Oostra BA, Pols HA, van Duijn CM. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- 26.Deal C, Ma J, Wilkin F, Paquette J, Rozen F, Ge B, Hudson T, Stampfer M, Pollak M. Novel promoter polymorphism in insulin-like growth factor-binding protein-3: correlation with serum levels and interaction with known regulators. J Clin Endocrinol Metab. 2001;86:1274–1280. doi: 10.1210/jcem.86.3.7280. [DOI] [PubMed] [Google Scholar]
- 27.Wong HL, Delellis K, Probst-Hensch N, Koh WP, Van Den Berg D, Lee HP, Yu MC, Ingles SA. A new single nucleotide polymorphism in the insulin-like growth factor I regulatory region associates with colorectal cancer risk in singapore chinese. Cancer Epidemiol Biomarkers Prev. 2005;14:144–151. [PubMed] [Google Scholar]
- 28.Zhao R, Berho M, Nogueras J, Sands D, Weiss E, Wexner S, Giardiello FM, Cruz-Correa M. Positive correlation of insulin-like growth factor-II with proliferating cell index in patients with colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2005;14:1819–1822. doi: 10.1158/1055-9965.EPI-04-0803. [DOI] [PubMed] [Google Scholar]
- 29.Renehan AG, Jones J, Potten CS, Shalet SM, O’Dwyer ST. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer. 2000;83:1344–1350. doi: 10.1054/bjoc.2000.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 31.Winkler R, Delacroix L, Bensbaho K, Lambert S, Collette J, Hodzic D. IGF-II in primary human colorectal tumors: peptide level, activated promoters, parental imprinting and gene rearrangement. Horm Metab Res. 1999;31:148–154. doi: 10.1055/s-2007-978713. [DOI] [PubMed] [Google Scholar]
- 32.Tadokoro K, Fujii H, Inoue T, Yamada M. Polymerase chain reaction (PCR) for detection of ApaI polymorphism at the insulin like growth factor II gene (IGF2) Nucleic Acids Res. 1991;19:6967. doi: 10.1093/nar/19.24.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 34.Bacci S, Menzaghi C, Ercolino T, Ma X, Rauseo A, Salvemini L, Vigna C, Fanelli R, Di Mario U, Doria A, Trischitta V. The +276 G/T single nucleotide polymorphism of the adiponectin gene is associated with coronary artery disease in type 2 diabetic patients. Diabetes Care. 2004;27:2015–2020. doi: 10.2337/diacare.27.8.2015. [DOI] [PubMed] [Google Scholar]
- 35.Vasseur F, Lepretre F, Lacquemant C, Froguel P. The genetics of adiponectin. Curr Diab Rep. 2003;3:151–158. doi: 10.1007/s11892-003-0039-4. [DOI] [PubMed] [Google Scholar]
- 36.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Feik E, Baierl A, Hieger B, Führlinger G, Pentz A, Stättner S, Weiss W, Pulgram T, Leeb G, Mach K, Micksche M, Gsur A. Association of IGF1 and IGFBP3 polymorphisms with colorectal polyps and colorectal cancer risk. Cancer Causes Control. 2010;21:91–97. doi: 10.1007/s10552-009-9438-4. [DOI] [PubMed] [Google Scholar]
- 38.Wong HL, Delellis K, Probst-Hensch N, Koh WP, Van Den Berg D, Lee HP, Yu MC, Ingles SA. A new single nucleotide polymorphism in the insulin-like growth factor I regulatory region associates with colorectal cancer risk in Singapore Chinese. Cancer Epidemiol Biomarkers Prev. 2005;14:144–151. [PubMed] [Google Scholar]
- 39.Wong HL, Koh WP, Probst-Hensch NM, Van den Berg D, Yu MC, Ingles SA. Insulin-like growth factor-1 promoter polymorphisms and colorectal cancer: a functional genomics approach. Gut. 2008;57:1090–1096. doi: 10.1136/gut.2007.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slattery ML, Samowitz W, Curtin K, Ma KN, Hoffman M, Caan B, Neuhausen S. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1206–1214. [PubMed] [Google Scholar]
- 41.He B, Pan Y, Zhang Y, Bao Q, Chen L, Nie Z, Gu L, Xu Y, Wang S. Effects of genetic variations in the adiponectin pathway genes on the risk of colorectal cancer in the Chinese population. BMC Med Genet. 2011;12:94. doi: 10.1186/1471-2350-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin JK, Shen MY, Lin TC, Lan YT, Wang HS, Yang SH, Li AF, Chang SC. Distribution of a single nucleotide polymorphism of insulin-like growth factor-1 in colorectal cancer patients and its association with mucinous adenocarcinoma. Int J Biol Markers. 2010;25:195–199. [PubMed] [Google Scholar]
- 43.Le Marchand L, Kolonel LN, Henderson BE, Wilkens LR. Association of an exon 1 polymorphism in the IGFBP3 gene with circulating IGFBP-3 levels and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14:1319–1321. doi: 10.1158/1055-9965.EPI-04-0847. [DOI] [PubMed] [Google Scholar]
- 44.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 45.Winkler R, Delacroix L, Bensbaho K, Lambert S, Collette J, Hodzic D. IGF-II in primary human colorectal tumors: peptide level, activated promoters, parental imprinting and gene rearrangement. Horm Metab Res. 1999;31:148–154. doi: 10.1055/s-2007-978713. [DOI] [PubMed] [Google Scholar]
- 46.Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev. 2004;13:1022. [PubMed] [Google Scholar]
- 47.Satia JA, Tseng M, Galanko JA, Martin C, Sandler RS. Dietary patterns and colon cancer risk in Whites and African Americans in the North Carolina Colon Cancer Study. Nutr Cancer. 2009;61:179–93. doi: 10.1080/01635580802419806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler LM, Millikan RC, Sinha R, Keku TO, Winkel S, Harlan B, Eaton A, Gammon MD, Sandler RS. Modification by N-acetyltransferase 1 genotype on the association between dietary heterocyclic amines and colon cancer in a multiethnic study. Mutat Res. 2008;638:162–174. doi: 10.1016/j.mrfmmm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keku T, Millikan R, Worley K, Winkel S, Eaton A, Biscocho L, Martin C, Sandler R. 5,10-Methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol Biomarkers Prev. 2002;11:1611–1621. [PubMed] [Google Scholar]
- 50.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 51.Block G. Dietary guidelines and the results of food consumption surveys. Am J Clin Nutr. 1991;53:356S–357S. doi: 10.1093/ajcn/53.1.356S. [DOI] [PubMed] [Google Scholar]
- 52.Willet WC. Nutritional Epidemiology. New York: Oxford University Press; 1990. [Google Scholar]
- 53.Collet D. Modeling Survival Data in Medical Research. Chapman & Hall; Boca Raton: 1994. [Google Scholar]
- 54.Hernandez W, Grenade C, Santos ER, Bonilla C, Ahaghotu C, Kittles RA. IGF-1 and IGFBP-3 gene variants influence on serum levels and prostate cancer risk in African-Americans. Carcinogenesis. 2007;28:2154–2159. doi: 10.1093/carcin/bgm190. [DOI] [PubMed] [Google Scholar]
- 55.Schildkraut JM, Demark-Wahnefried W, Wenham RM, Grubber J, Jeffreys AS, Grambow SC, Marks JR, Moorman PG, Hoyo C, Ali S, Walther PJ. IGF1 (CA)19 repeat and IGFBP3 -202 A/C genotypes and the risk of prostate cancer in Black and White men. Cancer Epidemiol Biomarkers Prev. 2005;14:403–408. doi: 10.1158/1055-9965.EPI-04-0426. [DOI] [PubMed] [Google Scholar]
- 56.Takacs I, Koller DL, Peacock M, Christian JC, Hui SL, Conneally PM, Johnston CC, Jr, Foround T, Econs MJ. Sibling pair linkage and association studies between bone mineral density and the insulin-like growth factor I gene locus. J Clin Endocrinol Metab. 1999;84:4467–4471. doi: 10.1210/jcem.84.12.6179. [DOI] [PubMed] [Google Scholar]
- 57.Tran N, Bharaj BS, Diamandis EP, Smith M, Li BD, Yu H. Short tandem repeat polymorphism and cancer risk: influence of laboratory analysis on epidemiologic findings. Cancer Epidemiol Biomarkers Prev. 2004;13:2133–2140. [PubMed] [Google Scholar]
- 58.Ruchat SM, Loos RJ, Rankinen T, Vohl MC, Weisnagel SJ, Després JP, Bouchard C, Pérusse L. Associations between glucose tolerance, insulin sensitivity and insulin secretion phenotypes and polymorphisms in adiponectin and adiponectin receptor genes in the Quebec Family Study. Diabet Med. 2008;25:400–406. doi: 10.1111/j.1464-5491.2008.02396.x. [DOI] [PubMed] [Google Scholar]
- 59.Gu HF, Abulaiti A, Ostenson CG, Humphreys K, Wahlestedt C, Brookes AJ, Efendic S. Single nucleotide polymorphisms in the proximal promoter region of the adiponectin (APM1) gene are associated with type 2 diabetes in Swedish caucasians. Diabetes. 2004;53:S31–S35. doi: 10.2337/diabetes.53.2007.s31. [DOI] [PubMed] [Google Scholar]
- 60.Hong Y, Pedersen NL, Brismar K, Hall K, de Faire U. Quantitative genetic analyses of insulin-like growth factor I (IGF-I), IGF-binding protein-1, and insulin levels in middle-aged and elderly twins. J Clin Endocrinol Metab. 1996;81:1791–1797. doi: 10.1210/jcem.81.5.8626837. [DOI] [PubMed] [Google Scholar]
- 61.Kao PC, Matheny AP, Jr, Lang CA. Insulin-like growth factor-I comparisons in healthy twin children. J Clin Endocrinol Metab. 1994;78:310–312. doi: 10.1210/jcem.78.2.8106617. [DOI] [PubMed] [Google Scholar]
- 62.Verhaeghe J, Loos R, Vlietinck R, Herck EV, van Bree R, Schutter AM. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in cord serum of twins: genetic versus environmental regulation. Am J Obstet Gynecol. 1996;175:1180–1188. doi: 10.1016/s0002-9378(96)70025-x. [DOI] [PubMed] [Google Scholar]
- 63.The International HapMap 3 Consortium. Integrating common and rare genetic variation in diverse human populations. Nature. 467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Insulin-like growth factor polymorphisms and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1204–1211. doi: 10.1158/1055-9965.EPI-04-0695. [DOI] [PubMed] [Google Scholar]
- 65.Giovannucci E, Haiman CA, Platz EA, Hankinson SE, Pollak MN, Hunter DJ. Dinucleotide repeat in the insulin-like growth factor-I gene is not related to risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2002;11:1509–1510. [PubMed] [Google Scholar]
- 66.Hoyo C, Grubber J, Denmark-Wahnefried W, Lobaugh B, Jeffreys AS, Grambow SC, Marks JR, Keku TO, Walther PJ, Shildkraut JM. Predictors of variation in serum IGF-I and IGFBP-3 levels in healthy African American and White men. J Natl Med Assoc. 2009;101:711–716. doi: 10.1016/s0027-9684(15)30981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoyo C, Grubber J, Demark-Wahnefried W, Marks JR, Freedland SJ, Jeffreys AS, Grambow SC, Wenham RM, Walther PJ, Schildkraut JM. Grade-specific prostate cancer associations of IGF1 (CA)19 repeats and IGFBP3-202A/C in blacks and whites. J Natl Med Assoc. 2007;99:718–722. [PMC free article] [PubMed] [Google Scholar]
- 68.Chen HY, Chan HIS, Sham ALK, Leung VHK, Ma SL, HOSC Haplotype effect in the IGF1 promoter accounts for the association between microsatellite and serum IGF1 concentration. Clin Endocrinol. 2011;74:520–527. doi: 10.1111/j.1365-2265.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs CS, Goldberg RM, Sargent DJ, Meyerhardt JA, Wolpin BM, Green EM, Pitot HC, Pollak M. Plasma insulin-like growth factors, insulin-like binding protein-3, and outcome in metastatic colorectal cancer: results from intergroup trial N9741. Clin Cancer Res. 2008;14:8263–8269. doi: 10.1158/1078-0432.CCR-08-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallego R, Codony-Serval J, Garcia-Albeniz X, Carcereny E, Longaron R, Oliveras A, Tosca M, Auge JM, Gascon P, Maurel J. Serum IGF-1, IGFBP-3, and matrix metalloproteinase-7 levels and acquired chemo-resistance in advanced colorectal cancer. Endocr Relat Cancer. 2009;16:311–317. doi: 10.1677/ERC-08-0250. [DOI] [PubMed] [Google Scholar]
- 71.Cruz-Correa M, Cui H, Giardiello FM, Powe NR, Hylind L, Robinson A, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Feinberg AP. Loss of imprinting of insulin growth factor II gene: a potential heritable biomarker for colon neoplasia predisposition. Gastroenterology. 2004;126:964–970. doi: 10.1053/j.gastro.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 72.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 73.MacInnis RJ, English DR, Hopper JL, Haydon AM, Gertig DM, Giles GG. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2004;13:553–559. [PubMed] [Google Scholar]
- 74.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 75.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte- secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 76.Filippi E, Sentinelli F, Trischitta V, Romeo S, Arca M, Leonetti F, Di Mario U, Baroni MG. Association of the human adiponectin gene and insulin resistance. Eur J Hum Genet. 2004;12:199–205. doi: 10.1038/sj.ejhg.5201120. [DOI] [PubMed] [Google Scholar]
- 77.Kaklamani VG, Wisinski KB, Sadim M, Gulden C, Do A, Offit K, Baron JA, Ahsan H, Mantzoros C, Pasche B. Variants of the adiponectin (ADIPOQ) and adiponectin receptor 1 (ADIPOR1) genes and colorectal cancer risk. JAMA. 2008;300:1523–1531. doi: 10.1001/jama.300.13.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menzaghi C, Ercolino T, Di Paola R, Berg AH, Warram JH, Scherer PE, Trischitta V, Doria A. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51:2306–2312. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- 79.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 80.Vionnet N, Hani EH, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Lepretre F, Lecoeur F, Gallina P, Zekiri L, Dina C, Froquel P. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21–q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 82.Lukanova A, Soderberg S, Kaaks R, Jellum E, Stattin P. Serum adiponectin is not associated with risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;15:401–402. doi: 10.1158/1055-9965.EPI-05-0836. [DOI] [PubMed] [Google Scholar]
- 83.Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, Boutin P, Vaxillaire M, Lepretre F, Dupont S, Hara K, Clement K, Bihain B, Kadowaki T, Froquel P. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 84.Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K, Kadowaki H, Hagura R, Akanuma Y, Yazaki Y, Nagai R, Taniyama M, Matsubara K, Yoda M, Nakano Y, Tomita M, Kimura S, Ito C, Froquel P, Kadowaki T. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–540. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 85.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovanucc EL, Fuchs CS. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2011;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546–553. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 87.Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, Hallmans G, Kaaks R. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003;107:89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 88.Manousos O, Souglakos J, Bosetti C, Tzonou A, Chatzidakis V, Trichopoulos D, Adami HO, Mantzoros C. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999;83:15–17. doi: 10.1002/(sici)1097-0215(19990924)83:1<15::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]


