Abstract
Introduction
We aim to better characterize the staining patterns of inverted papilloma (IP) with and without carcinoma by performing immunohistochemistry for p16, EGFR (Epidermal Growth Factor Receptor), p53, and Cyclin D1 antibodies on a large patient cohort.
Methods
One hundred and sixty-two IP specimens from 122 patients treated at the University of Michigan between 1996 and 2011. Twenty-two specimens contained carcinoma. Tumor was extracted for construction of two tissue microarrays and stained for p16, EGFR, p53, and Cyclin D1. Tumor staining intensity and percentage staining were scored.
Results
Mean percentage staining for IP and IP with carcinoma was 12% versus 7% for p16 (no statistical significance, NS), 20% versus 34% for EGFR (NS), 4% versus 24% for p53 (p<0.001), and 17% versus 21% for Cyclin D1 (NS). Benign disease was positive for p16 in 64%, EGFR in 50%, p53 in 30%, and Cyclin D1 in 76%. Inverted papilloma with carcinomatous degeneration was positive for p16 in 14%, EGFR in 71%, p53 in 62%, and Cyclin D1 in 76%. This is statistically significant for differences between IP and IP carcinoma for p16 and p53 staining only.
Conclusion
Important characteristic staining pattern for inverted papilloma with and without carcinoma are highlighted in this study. Unlike recent trends in HPV-related head and neck malignancies, low expression of p16 is a marker for malignancy in this series. Positive staining for p53 correlates with the development of carcinoma in inverted papilloma.
Keywords: Inverted papilloma, sinonasal carcinoma, p16, Cyclin D1, EGFR, p53
Introduction
Inverted papillomas (IP) of the sinonasal tract remain a topic of controversy among surgeons and pathologists regarding etiology, pathogenesis, and biological behavior. Representing between 0.4 to 4.7% of all sinonasal neoplasms, the overall incidence of these tumors is between 0.74 to 1.5 cases per 100,000 persons per year(1, 2). Risk factors for developing IP include outdoor and industrial occupation, but smoking and other inflammatory diseases such as allergic rhinitis and sinusitis are not considered etiologic factors(3). These tumors are known to degenerate into or simultaneously harbor carcinoma in some patients, oftentimes with aggressive growth patterns and intracranial or orbital extension that require radical resection. The current literature suggests a rate of malignancy of of 5–27%(4, 5), with an average of 10%(6, 7). Surgery remains the mainstay for treatment of inverted papilloma, and recurrence rates remains significant with a literature reported rate of 5.7–32%(8–10).
Perhaps secondary to its histologic appearance of hyperplastic Schneiderian mucosa enclosed in basement membrane that grows endophytically into the underlying stroma, the human papillomavirus (HPV) has been implicated in the development of inverted papilloma(11–15). However, definitive and objective data supporting this association remains sparse, and the true role of HPV in the pathogenesis of IP remains controversial. Detection rates for HPV vary widely in the literature with a range of 0–72 percent, with an average of about 25%(1, 11). Surrogate markers of HPV have been studied even less exhaustively, and no universally accepted immunohistochemical markers currently exist for this disease.
The retinoblastoma protein (pRB)/p16INK4a (p16) regulatory pathway is disrupted with HPV infection and is thus a good candidate for study in the pathogenesis of inverted papilloma. The protein p16 functions as a cyclin-dependent kinase inhibitor that shuts down Cyclin D1-dependent phosphorylation of the retinoblastoma gene (pRB), allowing dephosphorylation of pRb and cessation of cell cycle progression at the G1 to S check point. The retinoblastoma gene in its dephosphorylated state binds to the E2F family of transcription factors that up-regulate expression of proteins necessary for entry into the cell cycle. When a cell is infected with high risk human papillomavirus, the viral E7 protein binds to and inactivates pRb, activating E2F independently of cyclin dependent kinase. The functional inactivation of host pRb by the HPV E7 protein results in overexpression of p16, making p16 a reasonable surrogate marker for the presence of high risk human papillomavirus.
Another host regulatory pathway disrupted by HPV infection is cell-cycle control by the p53 tumor suppressor protein. E6 protein from the HPV DNA genome inactivates cellular regulatory protein p53 by associating with host E6 associated protein and ubiquinating host p53, marking this cell cycle regulatory protein for degradation(16). This process allows for unregulated cellular proliferation of epithelial cells and promotes oncogenesis. Epidermal Growth Factor Receptor (EGFR) can be similarly be overexpressed in HPV infected cells secondary to viral E5 gene product(17).
The purpose of this study is to better understand the biology of inverted papilloma by analyzing the staining pattern for p16, EGFR, Cyclin D1, and p53.
Methods
The University of Michigan Institutional Review Board (IRB) approved this study. Patients were identified from the University of Michigan pathology archive, using the search target “inverted papilloma” for exact matches. This study required a stringent pathology review of all patient blocks by dedicated head and neck pathologist (JM). To ensure consistency of pathology and patient reporting, other Schneiderian papillomas such as fungiform papillomas and cylindrical/oncocytic papillomas were excluded for data analysis. We included any inverted papilloma with or without dysplasia or carcinomatous degeneration treated at the University of Michigan between 1996 and 2011. One hundred and sixty-two IP specimens from 122 patients treated at the University of Michigan in the mentioned time period were studied. Charts were reviewed for demographic data, follow-up duration, recurrence, and presence of malignancy.
Tissue microarray (TMA) was constructed to facilitate the study of immunohistochemistry for p16, EGFR, p53, and Cyclin D1. The technique for TMA construction has previously been methodically described(18), and the reader is referred to this reference for details of construction. Briefly, individual tumor blocks were cut and stained for hematoxylin and eosin if corresponding slides for the tumor block were not available from the pathology archives. The area of tumor on each slide was marked, and the corresponding area of the tumor block was cored for TMA construction.
For immunochemistry, TMA slides were deparaffinized and rehydrated. Antigen retrieval method varied depending on the antibody used. EGFR: pepsin, Life technologies, 10 minutes at 37°C as per manufacture instructions. The standard citrate buffer was used for p53 and Cyclin D1 (25minutes; 92°C, cooled at room temperature for 25 minutes). For p16, CINtec p16INK4a Histology kit, mtm laboratories, Westborough, MA, kit instructions were followed. For all slides, peroxidase was quenched with Peroxidase Block. All slides were also blocked with casein for 30 minutes at room temperature. Primary antibody, EGFR/31G7 (Life Technologies, CARLSBAD CA), p53/D01 and Cyclin D1/Clone SP4 (Thermo Scientific, fremont, CA), and p16 (CINtec p16INK4a Histology kit, mtm laboratories, Westborough, MA) incubated for 1 hr. and probed with EnVision+System-HRP (DAKO) for p53, Cyclin D1 and EGFR. For p16 manufactures kit reagents were used.
Antibody binding was scored on a scale of 1 to 4 for all antibodies: Grade 1 for less than 5% staining, grade 2 for 5 to 20%, grade 3 for 21 to 50%, and grade 4 for greater than 50%. Intensity was scored as 1 for no staining, 2 for low intensity, 3 for moderate intensity, and 4 for high intensity. Proportion and intensity of staining were scored by a pathologist (JBM) who was blinded to the clinical outcome.
SPSS version 19 was used for all statistical calculation and analysis. The product limit method of Kaplan and Meier was used to calculate rates of local regional control, overall survival, and disease specific survival.
Results
One-hundred and forty six patients were diagnosed with inverted papilloma during the study period. One-hundred and sixty two pathology blocks were available for study from 147 patients, and this formed our study group for immunohistochemical analysis. Twenty-two specimens (13.6%) contained carcinoma. Among this study group, 111 patients were male. The cohort consisted of 112 Caucasians, 27 Blacks, 7 Asians, and 1 Hispanic patient.
P16
Eight samples were unsatisfactory for study, resulting in 154 total specimens. 104/154 (67.5%) showed some degree of staining for p16, with an average stain area of 11.3% (Table 1). The staining pattern of p16 is nuclear and cytoplasmic (Figure 1). Benign disease was positive for p16 in 64% versus 14% for IP with carcinoma (P<0.001) (Table 2). Mean percentage staining for IP and IP with carcinoma was 12% versus 7% for p16 (no statistical significance, NS) (Table 3).
Table 1.
Overall stain results of all samples based on intensity (category I=no staining, II=weak staining, III=moderate staining, IV=strong staining) and percent staining (category I for <5% staining, category II for 5–20% staining, category III for 21–50% staining, and category IV for >50% staining).
| p16 | EGFR | p53 | Cyclin D1 | |
|---|---|---|---|---|
| no staining (I) | 50 | 70 | 75 | 31 |
| weak staining (II) | 0 | 13 | 0 | 33 |
| moderate staining (III) | 14 | 39 | 1 | 27 |
| strong staining (IV) | 90 | 34 | 80 | 66 |
| <5% staining (I) | 67 | 72 | 98 | 37 |
| 5–20% staining (II) | 63 | 29 | 42 | 66 |
| 21–50% staining (III) | 19 | 29 | 13 | 48 |
| >50% staining (IV) | 5 | 26 | 3 | 6 |
| Average % staining | 11.3 | 21.7 | 7.5 | 17.3 |
| Total satisfactory samples | 154 | 156 | 156 | 157 |
Figure 1.
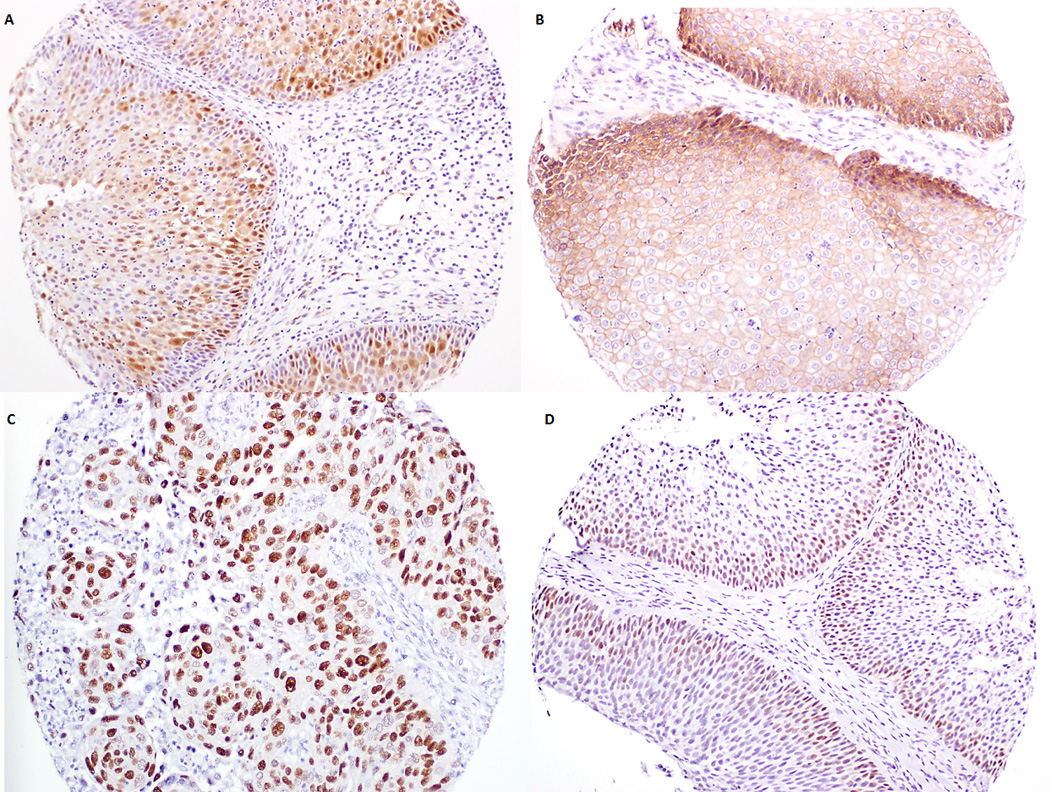
Stain patterns for biomarkers used. A. p16 staining is cytoplasmic and nuclear. This inverted papilloma (IP) specimen was graded 4 for intensity and 90% staining surface area. B. EGFR staining is cytoplasmic. This IP specimen was graded 4 for intensity and 100% for staining surface area. C. p53 staining nuclear. This IP specimen harbors squamous cell carcinoma and graded 4 for intensity and 100% for staining surface area. D. Cyclin D1 staining is also nuclear. This specimen of inverted papilloma was graded as 3 for intensity and 30% for staining surface area.
Table 2.
Number and percentage positive staining for p16, EGFR, p53, and Cyclin D1 based on benign versus inverted papilloma with carcinogenesis. NS=no statistical significance.
| Benign IP | IP with carcinoma |
Significance | |
|---|---|---|---|
| p16 + (%) | 85/133 (64%) | 3/21 (14%) | p<0.001 |
| EGFR+ (%) | 68/135 (50%) | 15/21 (71%) | NS |
| P53+ (%) | 45/135 (30%) | 13/21 (62%) | p<0.001 |
| Cyclin D1+ (%) | 104/136 (76%) | 16/21 (76%) | NS |
Table 3.
Average stain percentage for p16, EGFR, p53, and Cyclin D1for benign inverted papilloma versus IP with carcinoma. NS=no statistical significance.
| Benign IP | IP with carcinoma |
Significance | |
|---|---|---|---|
| p16 | 12% | 7% | NS |
| EGFR | 20% | 34% | NS |
| p53 | 4% | 24% | p<0.001 |
| Cyclin D1 | 17% | 21% | NS |
EGFR
Six samples were unsatisfactory for study, resulting in 156 total specimens. 86/156 (55%) showed some degree of staining for EGFR, with an average stain area of 21.7% (Table 1). The staining pattern of EGFR is membranous (Figure 1). Inverted papilloma harboring malignancy stained positive for EGFR in a higher percentage of specimens (71% versus 50%), but this difference was not statistically significant (Table 2). Mean percentage staining for IP and IP with carcinoma was 20% versus 34% (NS) (Table 3).
P53
Six samples were unsatisfactory for study, resulting in 156 total specimens. 81/156 (52%) showed some degree of staining for p53, with an average stain area of 7.5% (Table 1). The staining pattern of p53 is nuclear (Figure 1). Positive staining for p53 biomarker was found to be present in 30% of benign specimens versus 62% in specimens harboring malignancy (p<0.001) (Table 2). Mean percentage staining for IP and IP with carcinoma was 4% versus 24% (NS) (Table 3).
Cyclin D1
Five samples were unsatisfactory for study, resulting in 157 total specimens. 126/157 (80%) showed some degree of staining for Cyclin D1, with an average stain area of 17.3% (Table 1). Similar to p53 staining, Cyclin D1 staining is nuclear (Figure 1). Specimens with IP only and specimens with malignancy stained positive for Cyclin D1 in 76% (NS) (Table 2). Whereas the stain intensity for p16, EGFR, and p53 for positive-stain specimens were almost all moderate to strong, the stain intensity for Cyclin D1 positive samples varied widely. For Cyclin D1, the mean percentage staining for IP and IP with carcinoma was 17% versus 21% (NS) (Table 3).
Discussion
Characteristic staining patterns and reactivity are demonstrated on a large cohort of patients in this study for p16, EGFR, p53, and Cyclin D1. Overall, p16 staining was significantly more likely to be positive for benign disease, while p53 staining was more likely to be positive for IP with malignant degeneration. A higher percentage of inverted papilloma specimens with malignancy showed some degree of staining for EGFR compared to benign IP, but this difference did not reach statistical difference. We did not find any difference in overall staining patterns between benign and malignant disease for Cyclin D1. However, it is noted that a significant portion of both benign and malignant disease do demonstrate immunoreactivity to Cyclin D1 antibodies.
The literature is sparse on studies using p16 as a surrogate marker for HPV detection for inverted papilloma. Allende et al.(19) and Shah et al.(20) have investigated the use of p16 as a marker for HPV presence in two separate studies, but these authors reported contrasting results. Shah et al. reported that that 87.5% of HPV positive specimens by PCR were positive for p16, while 90.9% of HPV negative specimens also stained positive for p16. This study was limited by small patient population (n=26 patients) and used a combination of less sensitive PCR and biotinyl-tyramide-based chromogenic in situ hybridization for HPV detection. The high rates of p16 staining is opposite of a report by Allende et al., where 4/7 inverted papilloma were focally positive for p16, 2/6 dysplastic lesions showed patchy staining for p16, and 2/7 carcinomas were positive with p16. Their study did not find HPV in any specimens.
Our study is the first to statistically analyze and demonstrate a significantly higher degree of staining for p16 in IP than IP with malignancy. Our results suggest that p16 expression is usually not lost during the development of inverted papilloma. In the process of carcinogenesis, the p16 gene is more likely to be inactivated. This finding parallels non-HPV related head and neck cancer, where loss of p16 expression is one of the most common oncogene alterations secondary to loss of chromosomal region 9p21(21, 22). The progression to malignancy in inverted papilloma may share similar pathways or gene alterations seen in the development of other head and neck malignancies, but the exact mechanisms requires further study.
Because inactivation of retinoblastoma protein by high risk HPV E7 protein leads to p16 overexpression, p16 positivity is considered a useful surrogate marker for the presence of high risk HPV in head and neck cancer(23–25). The validity of p16 staining as evidence for HPV has not been shown in inverted papilloma. While this report does not specifically address HPV presence directly via in situ hybridization of PCR studies, the low average staining by surface area for all inverted papillomas in this study (12% for benign disease and 7% for IP with carcinoma) is significantly less than the diffuse positive staining seen in HPV related head and neck cancer(23, 24,26–28). One study by Lewis et al.(26) suggests 75% staining as a suitable cutoff for defining HPV positivity, and few of our specimens would meet this criteria. At this time, it is not possible to delineate whether the p16 staining detected in inverted papilloma seen in this study is a HPV related phenomenon or not. Because other factors such as abnormalities of the retinoblastoma protein or deletion and methylation of the p16 locus can also lead to p16 overexpression, p16 staining can be nonspecific.
EGFR is studied in our analysis because it can also be altered by HPV infection. The E5 gene protein from HPV DNA is a small hydrophobic protein consisting of 83 amino acids that localizes to the cell membrane, Golgi apparatus, and endosome. E5 enhances the activation of epidermal growth factor receptor (EGFR) and its downstream signal transduction pathways, leading to increased mitogenic activity(17). Proposed mechanisms for HPV E5 upregulation of EGFR include the EGFR-E5 protein complex which affects EGFR activity, E5 inhibition of endosome acidification which inhibits degradation of endocytic EGFR, and E5 inhibition of trafficking from early to late endosomes to delay EGFR degradation. Abnormal activation is seen in many epithelial malignancies such as head and neck cancer and is associated with poorer outcomes (29–31). For inverted papilloma, we found a trend for higher EGFR staining for IP with malignant degeneration. The difference in EGFR staining between IP with carcinoma and IP alone parallels what is seen in the development of epithelial malignancies but did not reach statistical significance. Chao et al.(32) also reported high percentage of EGFR staining in inverted papilloma specimens and low staining in polyp and inferior turbinate tissue, but the study did not perform statistical calculation to asses these differences.
Lastly, this study suggests that p53 alteration is important in the progression of inverted papilloma to malignant disease. IP with carcinoma stains positive for p53 at more than twice the frequency of IP alone (62% versus 30%). Compared to the low average percent stain by surface area in IP alone (4%), samples with malignancy showed significantly higher percentage area of positive staining (24%). In a study of 30 patients with Schneiderian papillomas, including 3 patients with either dysplasia or carcinoma, Mirza et al.(13) found 30% of samples to be p53 positive by immunochemistry. Positive staining for p53 in this study correlated to a 19% increase in the likelihood of malignancy at surgery. The difference in p53 staining between benign and malignant disease is higher in our cohort of patients. Franzmann et al.(33) also reported an overexpression of p53 in carcinomas occurring in sinonasal papillomas but not in the benign tumors of the sinonasal mucosa. Staining for p53 is also low for benign IP in a recent report by Sham et al.(34). Our study is congruent with these reports regarding the close relationship between p53 staining and malignancy.
Conclusion
Important characteristic staining patterns for inverted papilloma with and without carcinoma are highlighted in this study. Unlike recent trends in HPV-related head and neck malignancies, low expression of p16 is a marker for malignancy in this series. Positive staining for p53 correlates with the development of carcinoma. This study indicates that the pathogenesis of benign IP is from a non-p53 dependent pathway, while mutations in p53 likely play a larger role in the process of malignant degeneration.
Footnotes
The authors have no financial interests, disclosures or conflicts of interest regarding the content of this original manuscript.
References
- 1.Pensak ML. Controversies in otolaryngology. New York: Thieme; 2001. p. 476. xic. [Google Scholar]
- 2.Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002 Mar;15(3):279–297. doi: 10.1038/modpathol.3880524. PubMed PMID:11904343. Epub 2002/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 3.Sham CL, Lee DL, van Hasselt CA, Tong MC. A case-control study of the risk factors associated with sinonasal inverted papilloma. Am J Rhinol Allergy. 2010 Jan-Feb;24(1):e37–e40. doi: 10.2500/ajra.2010.24.3408. PubMed PMID:20109321. Epub 2010/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 4.Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995 Feb;105(2):178–183. doi: 10.1288/00005537-199502000-00013. PubMed PMID:8544600. Epub 1995/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 5.Lawson W, Ho BT, Shaari CM, Biller HF. Inverted papilloma: a report of 112 cases. Laryngoscope. 1995 Mar;105(3 Pt 1):282–288. doi: 10.1288/00005537-199503000-00011. PubMed PMID:7877417. Epub 1995/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 6.Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007 Sep;121(9):857–864. doi: 10.1017/S002221510700624X. PubMed PMID:17319993. Epub 2007/02/27. eng. [DOI] [PubMed] [Google Scholar]
- 7.von Buchwald C, Bradley PJ. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg. 2007 Apr;15(2):95–98. doi: 10.1097/MOO.0b013e3280803d9b. PubMed PMID:17413409. Epub 2007/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 8.Lombardi D, Tomenzoli D, Butta L, Bizzoni A, Farina D, Sberze F, et al. Limitations and complications of endoscopic surgery for treatment for sinonasal inverted papilloma: a reassessment after 212 cases. Head Neck. 2011 Aug;33(8):1154–1161. doi: 10.1002/hed.21589. PubMed PMID:20967873. Epub 2010/10/23. eng. [DOI] [PubMed] [Google Scholar]
- 9.Sham CL, Woo JK, van Hasselt CA, Tong MC. Treatment results of sinonasal inverted papilloma: an 18-year study. Am J Rhinol Allergy. 2009 Mar-Apr;23(2):203–211. doi: 10.2500/ajra.2009.23.3296. PubMed PMID:19401051. Epub 2009/04/30. eng. [DOI] [PubMed] [Google Scholar]
- 10.Carta F, Verillaud B, Herman P. Role of endoscopic approach in the management of inverted papilloma. Curr Opin Otolaryngol Head Neck Surg. 2011 Feb;19(1):21–24. doi: 10.1097/MOO.0b013e3283425213. PubMed PMID:21191294. Epub 2010/12/31. eng. [DOI] [PubMed] [Google Scholar]
- 11.Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008 Jun;2(2):49–59. doi: 10.1007/s12105-008-0048-3. PubMed PMID:20614323. Pubmed Central PMCID:2807546. Epub 2008/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck JC, McClatchey KD, Lesperance MM, Esclamado RM, Carey TE, Bradford CR. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol Head Neck Surg. 1995 Nov;113(5):558–563. doi: 10.1177/019459989511300506. PubMed PMID:7478645. Epub 1995/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 13.Mirza N, Montone K, Sato Y, Kroger H, Kennedy DW. Identification of p53 and human papilloma virus in Schneiderian papillomas. Laryngoscope. 1998 Apr;108(4 Pt 1):497–501. doi: 10.1097/00005537-199804000-00007. PubMed PMID:9546259. Epub 1998/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 14.Hwang CS, Yang HS, Hong MK. Detection of human papillomavirus (HPV) in sinonasal inverted papillomas using polymerase chain reaction (PCR) American journal of rhinology. 1998 Sep-Oct;12(5):363–366. doi: 10.2500/105065898780182499. PubMed PMID:9805538. Epub 1998/11/07. eng. [DOI] [PubMed] [Google Scholar]
- 15.Syrjanen K, Syrjanen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope. 2013 Jan;123(1):181–192. doi: 10.1002/lary.23688. PubMed PMID:23161522. Epub 2012/11/20. eng. [DOI] [PubMed] [Google Scholar]
- 16.Ishiji T. Molecular mechanism of carcinogenesis by human papillomavirus-16. The Journal of dermatology. 2000 Feb;27(2):73–86. doi: 10.1111/j.1346-8138.2000.tb02126.x. PubMed PMID:10721654. Epub 2000/03/18. eng. [DOI] [PubMed] [Google Scholar]
- 17.Pim D, Collins M, Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992 Jan;7(1):27–32. PubMed PMID:1311063. Epub 1992/01/01. eng. [PubMed] [Google Scholar]
- 18.Prince ME, Ubell ML, Castro J, Ogawa H, Ogawa T, Narayan A, et al. Tissue-preserving approach to extracting DNA from paraffin-embedded specimens using tissue microarray technology. Head & neck. 2007 May;29(5):465–471. doi: 10.1002/hed.20547. PubMed PMID:17252596. Epub 2007/01/26. eng. [DOI] [PubMed] [Google Scholar]
- 19.Allende DSHA, Batra P, Hunt JL. Dysplasia and carcinomas arising in Schneiderian papillomas, 20 years experience. Modern Pathology. 2008;21:231A. (Annual Meeting Abstracts) [Google Scholar]
- 20.Shah AA, Evans MF, Adamson CS, Peng Z, Rajendran V, Cooper K. HPV DNA is associated with a subset of Schneiderian papillomas but does not correlate with p16(INK4a) immunoreactivity. Head Neck Pathol. 2010 Jun;4(2):106–112. doi: 10.1007/s12105-010-0176-4. PubMed PMID:20405251. Pubmed Central PMCID:2878630. Epub 2010/04/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cairns P, Polascik TJ, Eby Y, Tokino K, Califano J, Merlo A, et al. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nature genetics. 1995 Oct;11(2):210–222. doi: 10.1038/ng1095-210. PubMed PMID:7550353. Epub 1995/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.van der Riet P, Nawroz H, Hruban RH, Corio R, Tokino K, Koch W, et al. Frequent loss of chromosome 9p21-22 early in head and neck cancer progression. Cancer research. 1994 Mar 1;54(5):1156–1158. PubMed PMID:8118798. Epub 1994/03/01. eng. [PubMed] [Google Scholar]
- 23.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jul 1;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. PubMed PMID:18474878. Pubmed Central PMCID:2744895. Epub 2008/05/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JM, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008 Jul 1;26(19):3138–3146. doi: 10.1200/JCO.2007.12.7597. PubMed PMID:18474879. Pubmed Central PMCID:2742158. Epub 2008/05/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. International journal of cancer Journal international du cancer. 2007 Apr 15;120(8):1731–1738. doi: 10.1002/ijc.22355. PubMed PMID:17236202. Epub 2007/01/20. eng. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JS, Jr, Chernock RD, Ma XJ, Flanagan JJ, Luo Y, Gao G, et al. Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol. 2012 Sep;25(9):1212–1220. doi: 10.1038/modpathol.2012.79. PubMed PMID:22596101. Epub 2012/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 27.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010 May 1;116(9):2166–2173. doi: 10.1002/cncr.25033. PubMed PMID:20186832. Epub 2010/02/27. eng. [DOI] [PubMed] [Google Scholar]
- 28.Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. The American journal of pathology. 2003 Mar;162(3):747–753. doi: 10.1016/S0002-9440(10)63871-0. PubMed PMID:12598309. Pubmed Central PMCID:1868106. Epub 2003/02/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. Journal of the National Cancer Institute. 1998 Jun 3;90(11):824–832. doi: 10.1093/jnci/90.11.824. PubMed PMID:9625170. Epub 1998/06/13. eng. [DOI] [PubMed] [Google Scholar]
- 30.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer research. 2002 Dec 15;62(24):7350–7356. PubMed PMID:12499279. Epub 2002/12/25. eng. [PubMed] [Google Scholar]
- 31.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Jul 15;21(14):2787–2799. doi: 10.1200/JCO.2003.01.504. PubMed PMID:12860957. Epub 2003/07/16. eng. [DOI] [PubMed] [Google Scholar]
- 32.Chao JC, Fang SY. Expression of epidermal growth factor receptor in the inverted papilloma and squamous cell carcinoma of nasal cavity. Eur Arch Otorhinolaryngol. 2008 Aug;265(8):917–922. doi: 10.1007/s00405-008-0582-3. PubMed PMID:18231801. Epub 2008/01/31. eng. [DOI] [PubMed] [Google Scholar]
- 33.Franzmann MB, Buchwald C, Jacobsen GK, Lindeberg H. Expression of p53 in normal nasal mucosa and in sinonasal papillomas with and without associated carcinoma and the relation to human papillomavirus (HPV) Cancer letters. 1998 Jun 19;128(2):161–164. doi: 10.1016/s0304-3835(98)00058-5. PubMed PMID:9683277. Epub 1998/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 34.Sham CL, To KF, Chan PK, Lee DL, Tong MC, van Hasselt CA. Prevalence of human papillomavirus, Epstein-Barr virus, p21, and p53 expression in sinonasal inverted papilloma, nasal polyp, and hypertrophied turbinate in Hong Kong patients. Head & neck. 2012 Apr;34(4):520–533. doi: 10.1002/hed.21772. PubMed PMID:21608063. Epub 2011/05/25. eng. [DOI] [PubMed] [Google Scholar]


