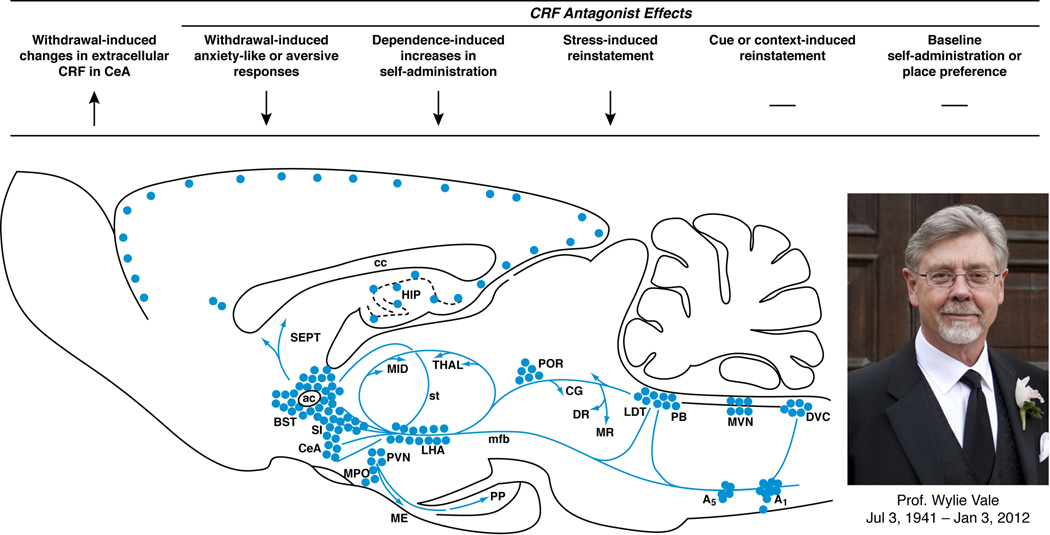
Figure 1. Brain CRF mediates the facilitation of compulsive-like drug use.
As shown in the sagittal brain schematic, corticotropin-releasing factor (CRF), first isolated by Professor Wylie Vale (photo), is expressed in neuronal cell bodies (filled circles) and projections (blue arrows) that subserve behavioral, autonomic and neuroendocrine responses to stress. As summarized from left-to-right by the arrows, CRF systems play an integral role in regulating the intersection between drug self-administration and stress systems. For example, drug or alcohol withdrawal elevates CRF activity in the central extended amygdala, including the central nucleus of the amygdala (CeA), leading to a negative emotional state that motivates resumption of and maintenance of drug-taking. Pharmacological studies with CRF antagonists show that increased CRF-CRF1 system activation underlies several withdrawal-induced behavioral phenotypes, including anxiety-like behavior, aversion, and elevated drug self-administration. CRF1 antagonists also reduce the effect of acute stressors on drug-related behaviors, including stress-induced reinstatement. In contrast, CRF1 antagonists do not alter non-stress mechanisms that reinstate drug-seeking, such as drug primes, cues or contexts, reflecting the distinct neuroanatomical substrates of relapse behavior. Blockade of CRF-CRF1 systems does not have intrinsic rewarding (or aversive) properties in place conditioning models and has little effect on baseline intake in nondependent individuals.