Abstract
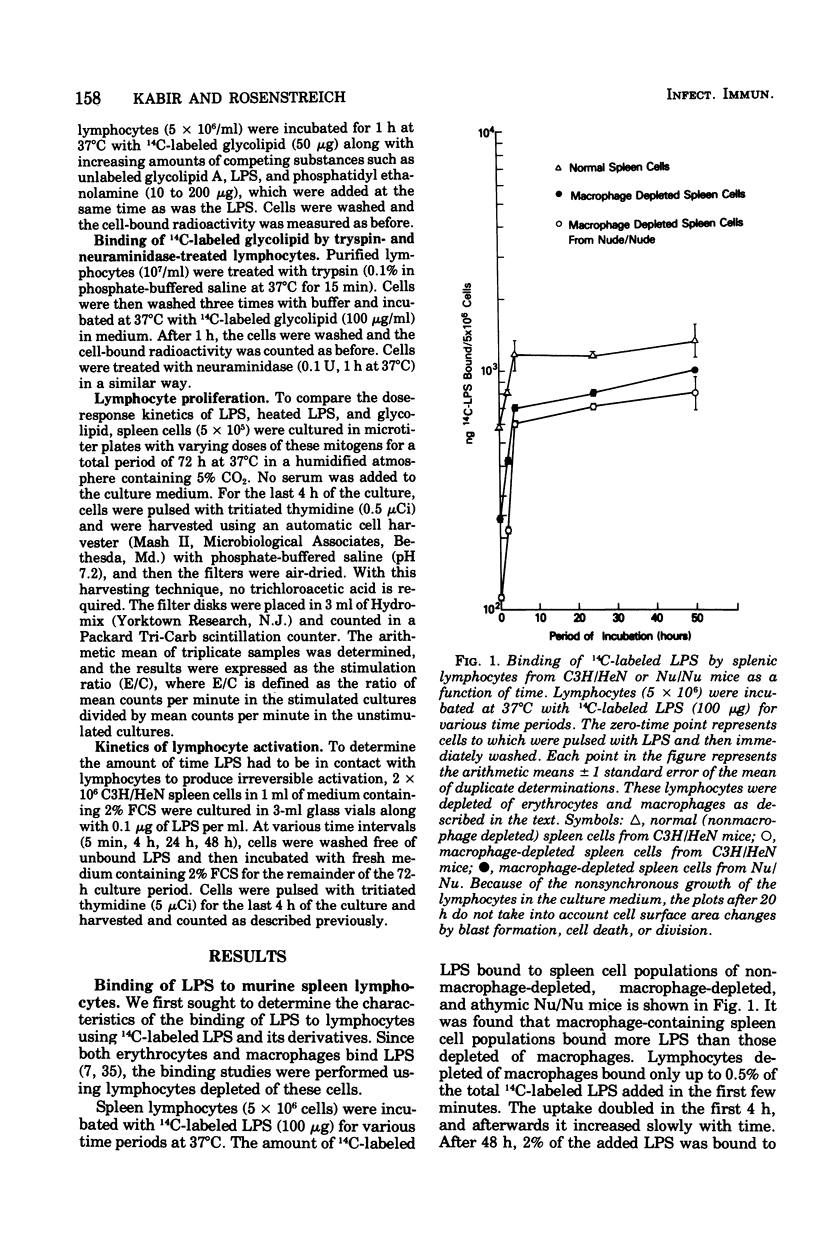
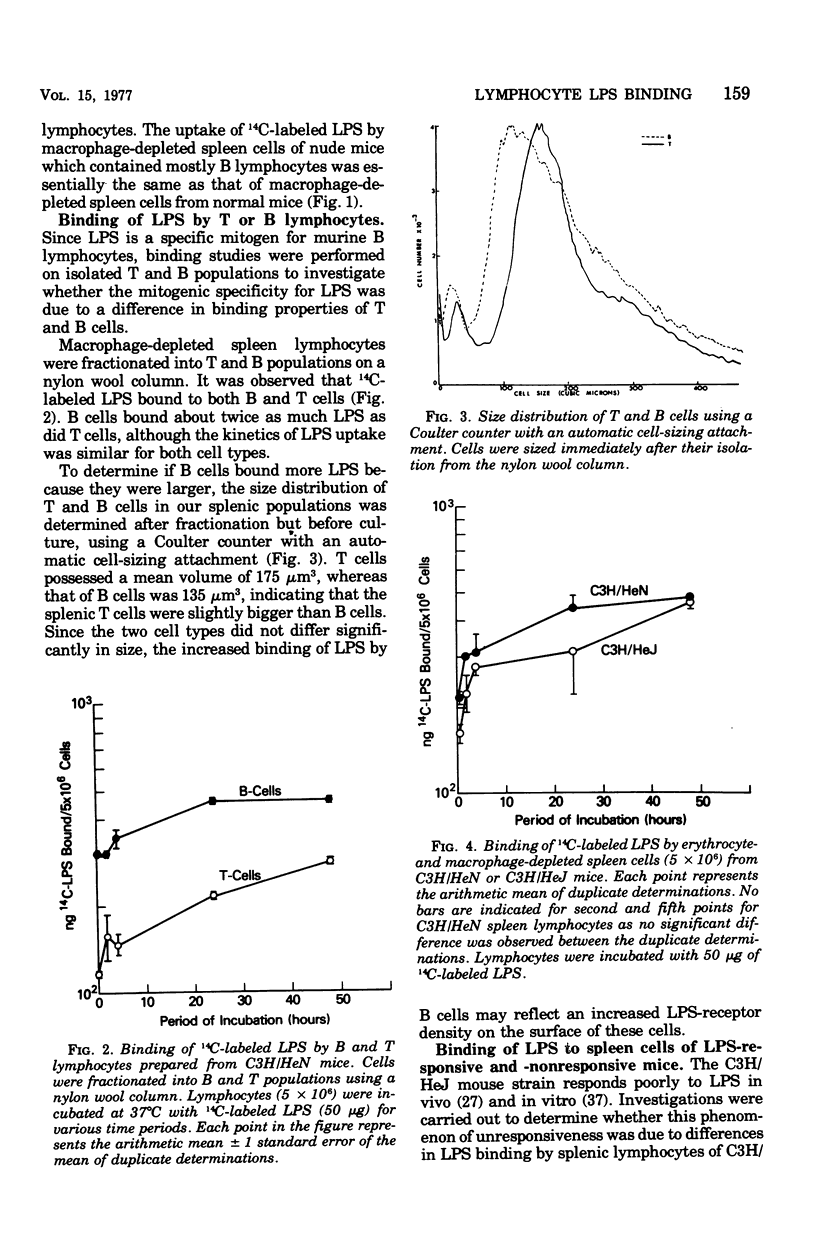
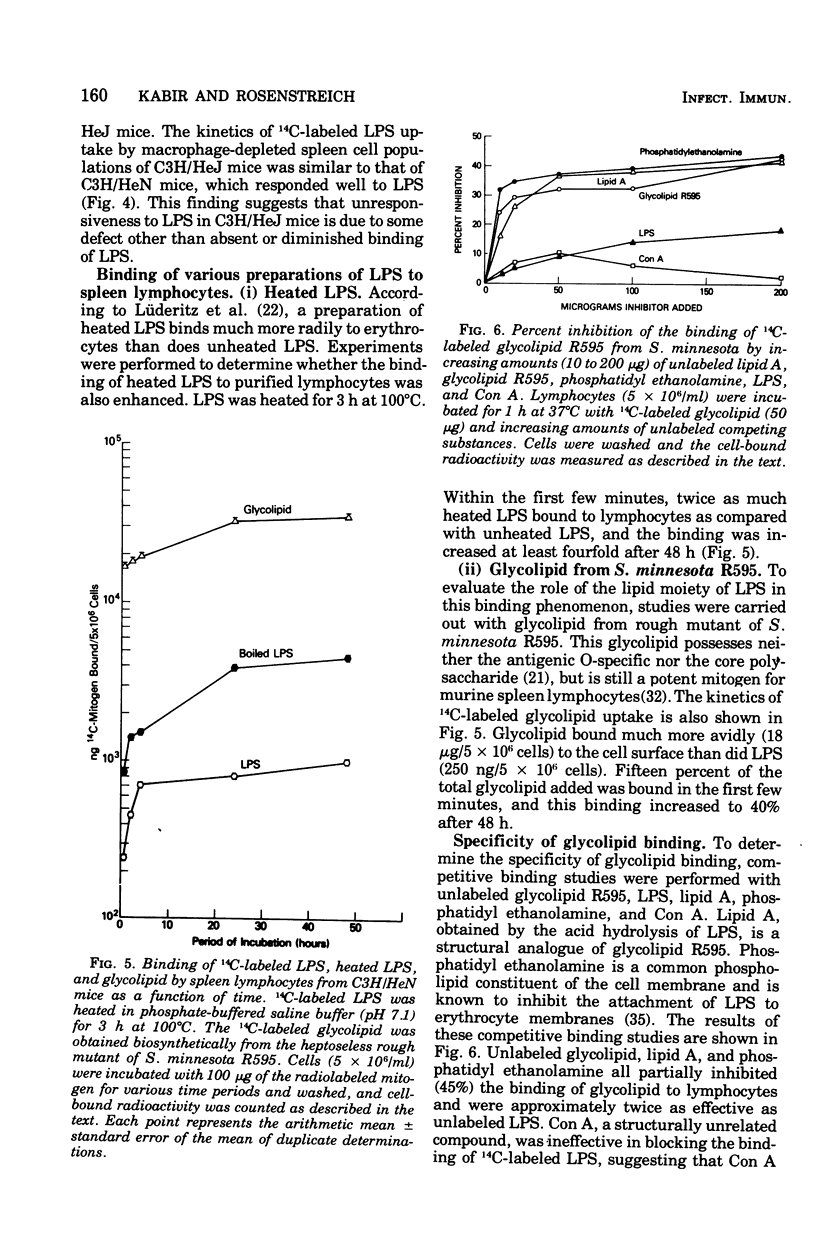
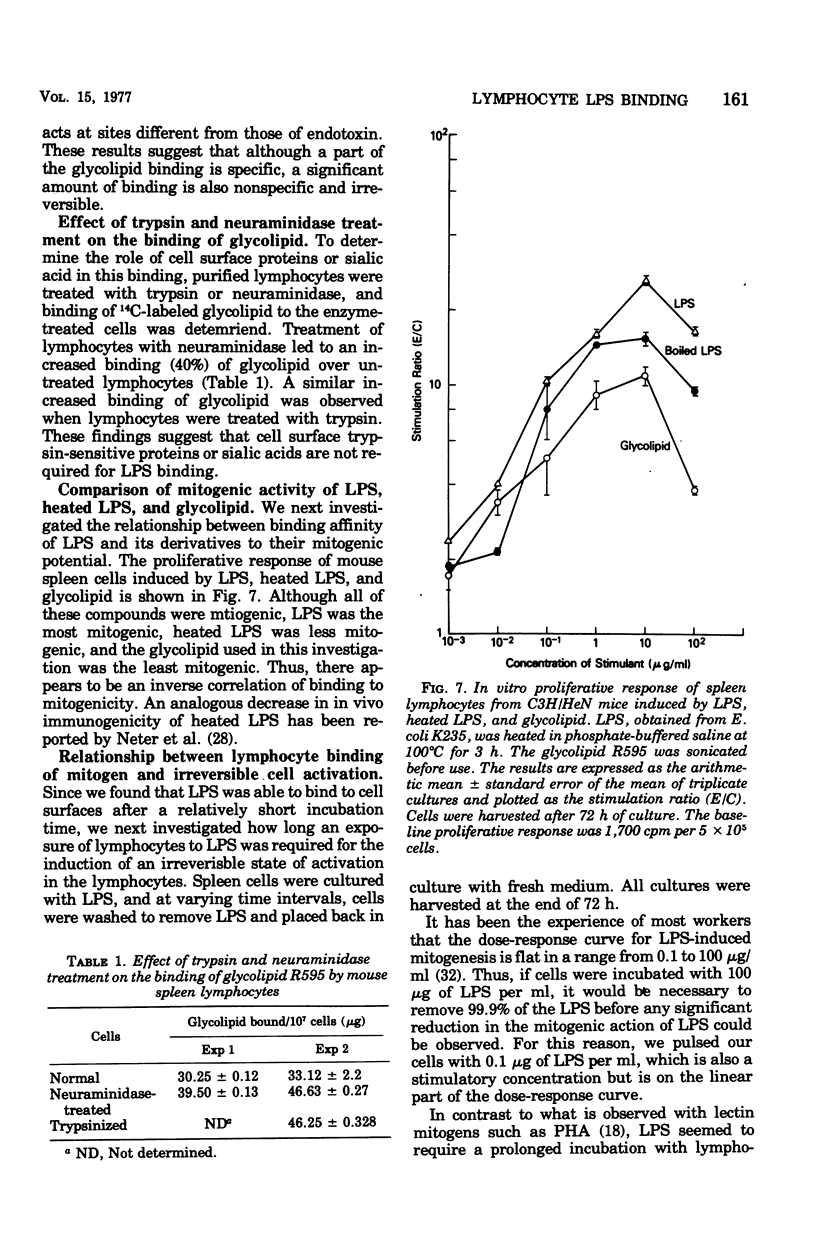
The early events in lipopolysaccharide (LPS)-induced B-cell activation were investigated by studying the binding of 14C-labeled LPS to murine lymphocytes in vitro. In these studies we utilized intrinsically labeled 14C-labeled LPS from Salmonella minnesota or the 14C-labeled glycolipid derived from the Re mutant of S. minnesota (R595). Bone marrow-derived (B) lymphocytes bound more LPS than did thymus-derived (T) lymphocytes. Binding of LPS to murine spleen lymphocytes from strain C3H/HeN was compared with the binding to spleen lymphocytes from strain C3H/HeJ, a strain resistant to certain biological activities of LPS including mitogenesis. Spleen cells from both strains bound LPS equally well, suggesting that unresponsiveness of C3H/HeJ mice to LPS is due to factors other than a defect in binding of LPS. LPS binding to cells appeared to be due to a nonspecific interaction between the lipid moiety of LPS and the lipid components of the cell membrane. Thus, the highly lipophilic, polysaccharide-deficient glycolipid from R595 bound at least 20 times better than did LPS. Furthermore, partial removal of cell surface proteins with trypsin or sialic acids with neuraminidase enhanced glycolipid binding, suggesting that binding is not through a protein- or sialic acid-containing receptor. The binding of glycolipid to lymphocytes was only partially specific since unlabeled glycolipid R595, lipid A, and LPS did not completely inhibit the uptake of 14C-labeled glycolipid R595. In addition, binding could be inhibited by a nonmitogenic phospholipid (phosphatidyl ethanolamine), which also is consistent with a nonspecific lipid-lipid interaction. Experiments were performed to determine the relationship of LPS binding to lymphocyte activation in the lymphocytes. The process of activation of lymphocytes by LPS was a slow one, since LPS was required to be present in culture for at least 24 h in order to obtain significant lymphocyte activation, suggesting that the amounts of LPS bound earlier are either quantitatively or qualitatively insufficient to irreversibly activate the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Osunkoya B. O., Takiguchi T., Smith R. T. The interactions of mitogens with lymphoid cells and the effect of neuraminidase on the cells' responsiveness to stimulation. Cell Immunol. 1972 Apr;3(4):590–605. doi: 10.1016/0008-8749(72)90121-9. [DOI] [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Bara J., Lallier R., Trudel M., Brailovsky C., Nigam V. N. Molecular models on the insertion of a Salmonella minnesota R-form glycolipid into the cell membrane of normal and transformed cells. Eur J Biochem. 1973 Jun 15;35(3):495–498. doi: 10.1111/j.1432-1033.1973.tb02864.x. [DOI] [PubMed] [Google Scholar]
- Benedetto D. A., Shands J. W., Jr, Shah D. O. The interaction of bacterial lipopolysaccharide with phospholipid bilayers and monolayers. Biochim Biophys Acta. 1973 Mar 16;298(2):145–157. doi: 10.1016/0005-2736(73)90346-5. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. Evidence for thymic dependence of PHA-reactive cells in spleen and lymph nodes and independence in bone marrow. J Immunol. 1971 Mar;106(3):835–841. [PubMed] [Google Scholar]
- Bona C., Robineaux R., Anteunis A., Heuclin C., Astesano A. Transfer of antigen from macrophages to lymphocytes. II. Immunological significance of the transfer of lipopolysaccharide. Immunology. 1973 May;24(5):831–840. [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Despont J. P., Abel C. A., Grey H. M. Sialic acids and sialyltransferases in murine lymphoid cells: indicators of T cell maturation. Cell Immunol. 1975 Jun;17(2):487–494. doi: 10.1016/s0008-8749(75)80052-9. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. The thymus and circulating lymphocytes of mice. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):69–85. doi: 10.1098/rspb.1970.0035. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Fisher R. I. Characterization of the lymphocyte surface receptors for Con A and PHA. J Immunol. 1975 Feb;114(2 Pt 1):710–714. [PubMed] [Google Scholar]
- Kay J. E. The role of the stimulant in the activation of lymphocytes by PHA. Exp Cell Res. 1969 Nov;58(1):185–188. doi: 10.1016/0014-4827(69)90134-7. [DOI] [PubMed] [Google Scholar]
- LUDERITZ O., WESTPHAL O., SIEVERS K., KROGER E., NETER E., BRAUN O. H. Uber die Fixation von P32-markiertem Lipopolysaccharid (Endotoxin) aus Escherichia coli an menschlichen Erythrocyten. Biochem Z. 1958;330(1):34–46. [PubMed] [Google Scholar]
- Lindahl-Kiessling K., Mattsson A. Mechanism of phytohemagglutinin (PHA) action. IV. Effect of some metabolic inhibitors on binding of PHA to lymphocytes and the stimulatory potential of PHA-pretreated cells. Exp Cell Res. 1971 Apr;65(2):307–312. doi: 10.1016/0014-4827(71)90006-1. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Nurminen M., Rietschel E. T., Rosenfelder G., Simon M., Westphal O. Lipid A: chemical structure and biological activity. J Infect Dis. 1973 Jul;128(Suppl):17–29. doi: 10.1093/infdis/128.supplement_1.s17. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Möller G., Andersson J., Pohlit H., Sjöberg O. Quantitation of the number of mitogen molecules activating DNA synthesis in T and B lymphocytes. Clin Exp Immunol. 1973 Jan;13(1):89–99. [PMC free article] [PubMed] [Google Scholar]
- Möller G., Coutinho A., Persson U. Mechanism of b-lymphocyte activation: failure to obtain evidence of a direct role of the Ig receptors in the triggering process. Scand J Immunol. 1975;4(1):37–52. doi: 10.1111/j.1365-3083.1975.tb02598.x. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Asselineau J., Mergenhagen S. E., Nowotny A. A synthetic glycolipid with B-cell mitogenic activity. J Exp Med. 1974 Nov 1;140(5):1404–1409. doi: 10.1084/jem.140.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Nowotny A., Chused T., Mergenhagen S. E. In vitro transformation of mouse bone-marrow-derived (B) lymphocytes induced by the lipid component of endotoxin. Infect Immun. 1973 Sep;8(3):406–411. doi: 10.1128/iai.8.3.406-411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman S. J. In vitro susceptibility of methicillin-resistant Staphylococcus aureus to sulfamethoxazole and trimethoprim. J Infect Dis. 1973 Nov;128(Suppl):543–p. doi: 10.1093/infdis/128.supplement_3.s543. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Adye J. C., Bezkorovainy A., Jirgensons B. Properties and activity of the lipopolysaccharide-receptor from human erythrocytes. Biochemistry. 1974 Mar 26;13(7):1379–1389. doi: 10.1021/bi00704a011. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- WESTPHAL O., NOWOTNY A., LUDERITZ O., HURNI H., EICHENBERGER E., SCHONHOLZER G. Die Bedeutung der Lipoid-Komponente (Lipoid A) für die biologischen Wirkungen bakterieller Endotoxine (Lipopolysaccharide). Pharm Acta Helv. 1958 Aug-Oct;33(8-10):401–411. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]
- Weber T. H., Lindahl-Kiessling K., Mattsson A., Alm G. V. Autoradiographic studies of lymphocytes stimulated in vitro with tritium labelled kidney bean leucoagglutinin. Life Sci I. 1972 Apr 1;11(7):343–350. doi: 10.1016/0024-3205(72)90060-4. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Craig W. A., Amidon G. L., Kunin C. M. Pharmacokinetics of trimethoprim and sulfamethoxazole in normal subjects and in patients with renal failure. J Infect Dis. 1973 Nov;128(Suppl):556–p. doi: 10.1093/infdis/128.supplement_3.s556. [DOI] [PubMed] [Google Scholar]


