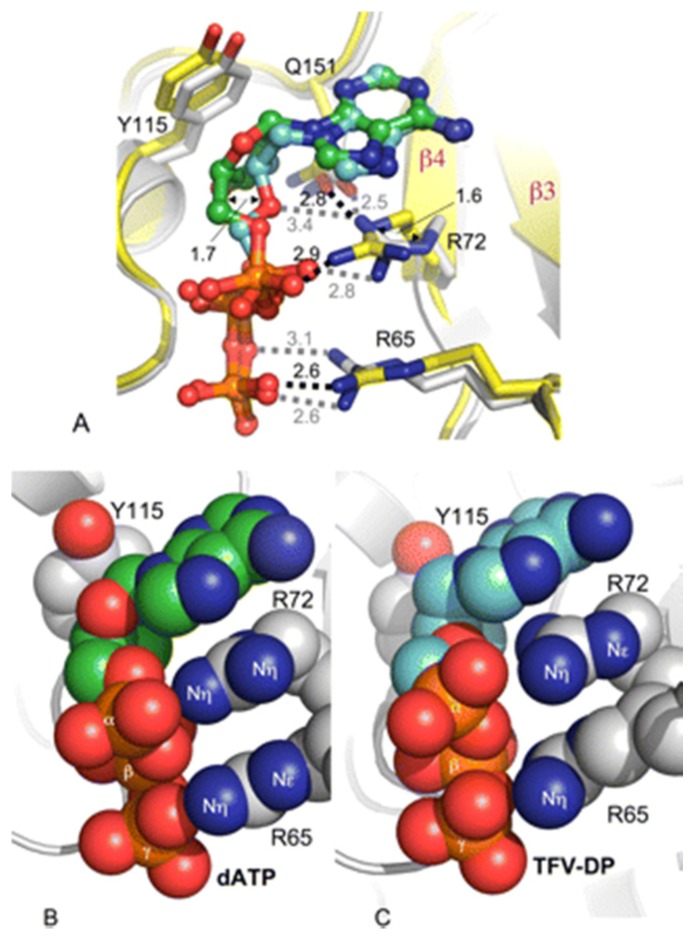
Figure 1.
Comparison of binding of tenofovir diphosphate (TFV-DP) and dATP to K65R RT·dsDNA. A, overlay of TFV-DP (TFV-DP (cyan) and RT (gray) and dATP (dATP (green) with RT (yellow)) shows that the structural difference between the inhibitor and the substrate at the deoxyribose moiety positions Arg72 and Arg65 side chains differently. The polar interactions are represented by dotted lines. Shown is stacking of the guanidinium plane of Arg65 and guanidinium plane of Arg72 and adenine base in K65R RT·dsDNA·dATP (B) and K65R RT·dsDNA·TFV-DP (C) ternary structures. Despite differences in the rotameric conformations of Arg65 and Arg72, the hydrophobic stacking is maintained in both structures. Reproduced from [29].