Figure 1.
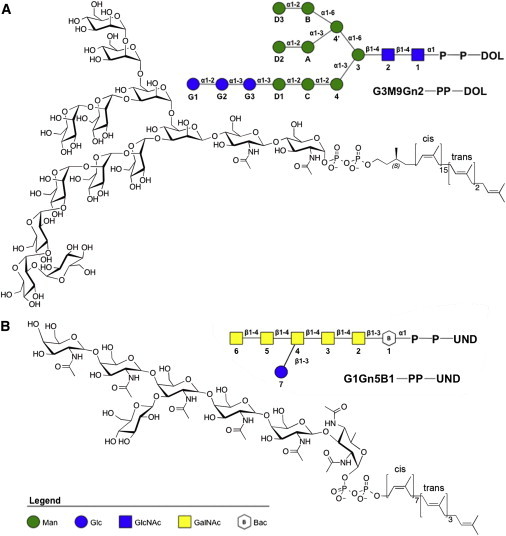
Chemical and schematic representations of the (A) eukaryotic and (B) bacterial LLOs used in this study. In the legend, Man: d-mannose; Glc: d-glucose; GlcNAc: N-acetyl-d-glucosamine; GalNAc: N-acetyl-d-galactosamine; Bac: d-bacillosamine. The oligosaccharide is covalently attached to a lipid chain by pyrophosphate. In (A), the oligosaccharide is composed of two GlcNAc residues joined to nine branching Man residues capped with three glucose residues. The lipid chain is composed of a saturated isoprenyl unit with an S chiral center, joined in series to 15 isoprenyl units with double bonds in cis configuration, followed by two isoprenyl units with trans double bonds, and a terminal isoprenyl unit. In (B), the oligosaccharide is composed of a Bac residue linked to five GalNAc residues, and a Glc residue attached to the third GalNAc. In the lipid chain, all isoprenyl units are unsaturated, the first seven units have cis double bonds and next three have trans with a terminal isoprenyl unit. Note that the anomeric center of the first sugar residue attached to pyrophosphate in both (A) eukaryotic and (B) bacterial LLOs is in the α configuration. To see this figure in color, go online.
