Abstract
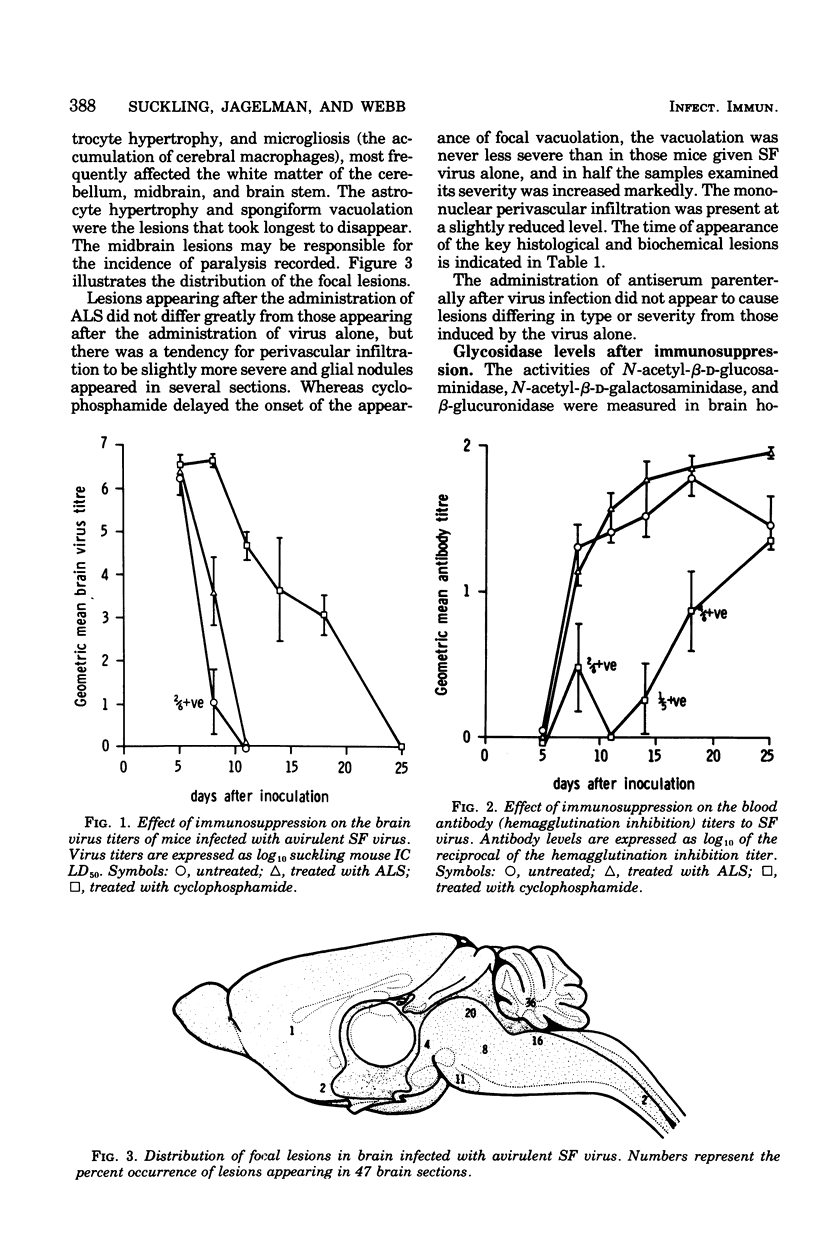
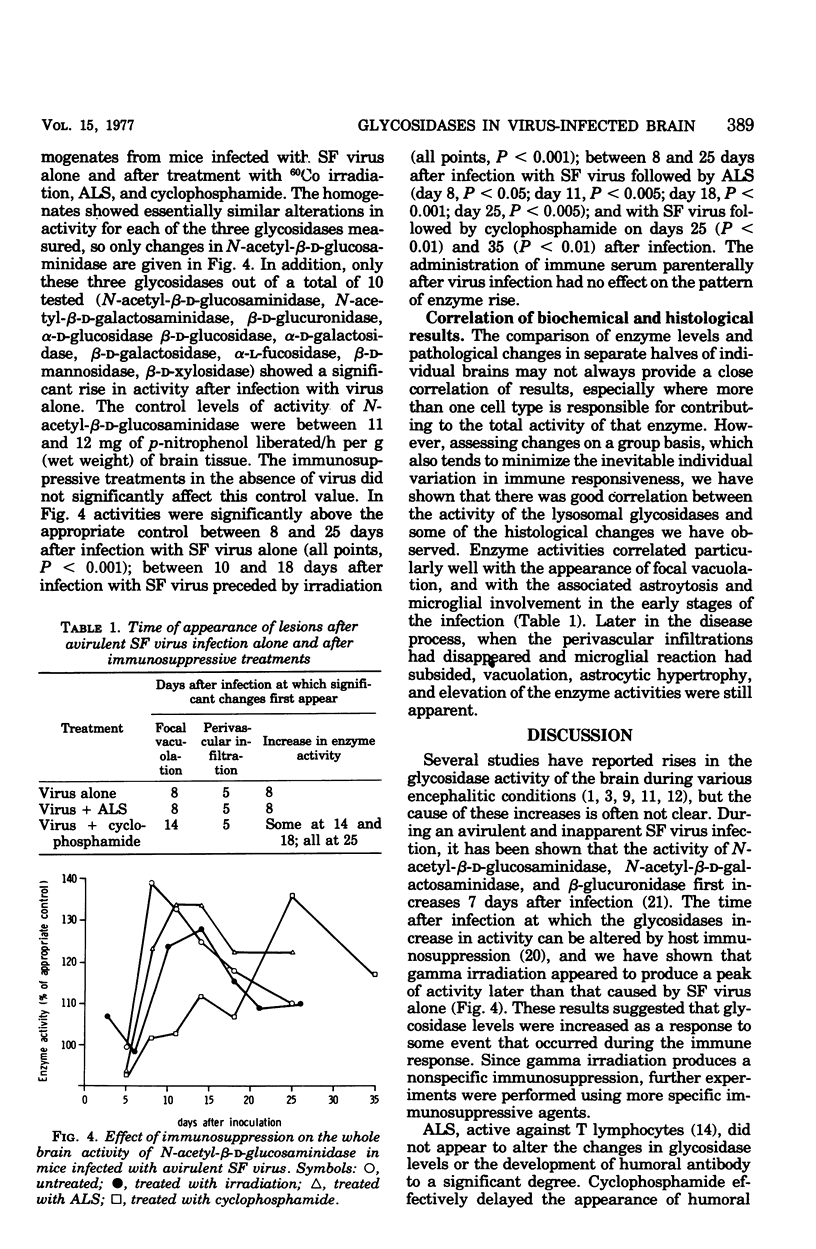
Mice infected with an avirulent strain of Semliki forest virus show an increase in the activity of some of the brain lysosomal glycosidases. The increase in activity of these enzymes has been correlated with the histological, virological, and serological changes that result from the infection in the presence and absence of immunosuppression. Semliki forest virus alone caused the development of a mild encephalitis with perivascular infiltration, microgliosis, astrocyte hypertrophy, and a focal spongiform encephalopathy, together with an increased activity of brain N-acetyl-beta-D-glucosaminidase and beta-glucuronidase. Antilymphocyte serum given after infection marginally affected the course of the disease. Cyclophosphamide markedly delayed the development of the spongy changes in the increase in enzyme activities, but not the perivascular infiltration. It is suggested that the increased activity of the lysosomal glycosidases studied may be linked both to the development of a successful immune response and to the focal spongiform changes produced by the infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arstila A. U., Riekkinen P., Rinne U. K., Laitinen L. Studies on the pathogenesis of multiple sclerosis. Participation of lysosomes on demyelination in the central nervous system white matter outside plaques. Eur Neurol. 1973;9(1):1–20. doi: 10.1159/000114197. [DOI] [PubMed] [Google Scholar]
- Asher D. M., Gibbs C. J., Jr, Gajdusek D. C. Pathogenesis of subacute spongiform encephalopathies. Ann Clin Lab Sci. 1976 Jan-Feb;6(1):84–103. [PubMed] [Google Scholar]
- Bowen D. M., Flack R. H., Martin R. O., Smith C. B., White P., Davison A. N. Biochemical studies on degenerative neurological disorders. I. Acute experimental encephalitis. J Neurochem. 1974 Jun;22(6):1099–1107. doi: 10.1111/j.1471-4159.1974.tb04342.x. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Cardella C. J., Davies P., Allison A. C. Immune complexes induce selective release of lysosomal hydrolases from macrophages. Nature. 1974 Jan 4;247(5435):46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- Chandler R. L., Smith K. Electron microscopic observations on scrapie in the mouse, with reference to glucosaminidase activity. Res Vet Sci. 1968 May;9(3):228–230. [PubMed] [Google Scholar]
- Chew-Lim M. Mouse encephalitis induced by avirulent Semliki forest virus. Vet Pathol. 1975;12(5-6):387–393. doi: 10.1177/0300985875012005-00605. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Reid H. W., Smith W. Louping-ill encephalomyelitis in the sheep. IV. Nature of the perivascular inflammatory reaction. J Comp Pathol. 1971 Oct;81(4):545–549. doi: 10.1016/0021-9975(71)90083-1. [DOI] [PubMed] [Google Scholar]
- Hirsch H. E., Parks M. E. Acid proteinases and other acid hydrolases in experimental allergic encephalomyelitis: pinpointing the source. J Neurochem. 1975 May;24(5):853–858. doi: 10.1111/j.1471-4159.1975.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Levey R. H., Medawar P. B. Nature and mode of action of antilymphocytic antiserum. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1130–1137. doi: 10.1073/pnas.56.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A., Wilson A. M., Dennis P. F. Further observations on histochemical changes in scrapie mouse brain. Comparison with experimental viral encephalitides. J Comp Pathol. 1968 Oct;78(4):489–498. doi: 10.1016/0021-9975(68)90048-0. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Santos G. W., Quinn R. P. Immunosuppressive drugs. Pharmacol Rev. 1970 Jun;22(2):189–247. [PubMed] [Google Scholar]
- McMartin D. N., Koestner A., Long J. F. Enzyme activities associated with the demyelinating phase of canine distemper. I. Beta-glucuronidase, acid and neutral proteinases. Acta Neuropathol. 1972;22(4):275–287. doi: 10.1007/BF00809239. [DOI] [PubMed] [Google Scholar]
- Medawar P. Review lecture. Immunosuppressive agents, with special reference to antilymphocytic serum. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):155–172. doi: 10.1098/rspb.1969.0086. [DOI] [PubMed] [Google Scholar]
- Millson G. C., Bountiff L. Glycosidases in normal and scrapie mouse brain. J Neurochem. 1973 Feb;20(2):541–546. doi: 10.1111/j.1471-4159.1973.tb12153.x. [DOI] [PubMed] [Google Scholar]
- Reid H. W., Doherty P. C., Dawson A. M. Louping-ill encephalomyelitis in the sheep. 3. Immunoglobulins in cerebrospinal fluid. J Comp Pathol. 1971 Oct;81(4):537–543. doi: 10.1016/0021-9975(71)90082-x. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Seamer J. H., Boulter E. A., Zlotnik I. Delayed onset of encephalitis in mice passively immunised against Semliki Forest virus. Br J Exp Pathol. 1971 Aug;52(4):408–414. [PMC free article] [PubMed] [Google Scholar]
- Suckling A. J., Bateman S., Webb H. E. Brain lysosomal glycosidase activity in mice during the immune response to an encephalitogenic virus infection. Biochem Soc Trans. 1976;4(2):326–328. doi: 10.1042/bst0040326. [DOI] [PubMed] [Google Scholar]
- Suckling A. J., Webb H. E., Chew-Lim M., Oaten S. W. Effect of an inapparent viral encephalitis on the levels of lysosomal glycosidases in mouse brain. J Neurol Sci. 1976 Sep;29(1):109–116. doi: 10.1016/0022-510x(76)90084-8. [DOI] [PubMed] [Google Scholar]
- Turk J. L. Evidence for a preferential effect of cyclophosphamide on B-cells. Proc R Soc Med. 1973 Aug;66(8):805–808. [PMC free article] [PubMed] [Google Scholar]
- WARREN J., WEIL M. L. Purification of certain viruses by use of protamine sulfate. Proc Soc Exp Biol Med. 1949 Dec;72(3):662–664. doi: 10.3181/00379727-72-17535. [DOI] [PubMed] [Google Scholar]
- Zlontnik I., Grant D. P., Carter G. B. Experimental infection of monkeys with viruses of the tick-borne encephalitis complex: degenerative cerebellar lesions following inapparent forms of the disease or recovery from clinical encephalitis. Br J Exp Pathol. 1976 Apr;57(2):200–210. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Grant D. P., Batter-Hatton D. Encephalopathy in mice following inapparent Semliki Forest Virus (S.F.V.) infection. Br J Exp Pathol. 1972 Apr;53(2):125–129. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Grant D. P., Carter G. B., Batter-Hatton D. Subacute sclerosing encephalitis in adult hamsters infected with Langat virus. Br J Exp Pathol. 1973 Feb;54(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Peacock S., Grant D. P., Batter-Hatton D. The pathogenesis of western equine encephalitis virus (W.E.E.) in adult hamsters with special reference to the long and short term effects on the C.N.S. of the attenuated clone 15 variant. Br J Exp Pathol. 1972 Feb;53(1):59–77. [PMC free article] [PubMed] [Google Scholar]


