Fig. 3.
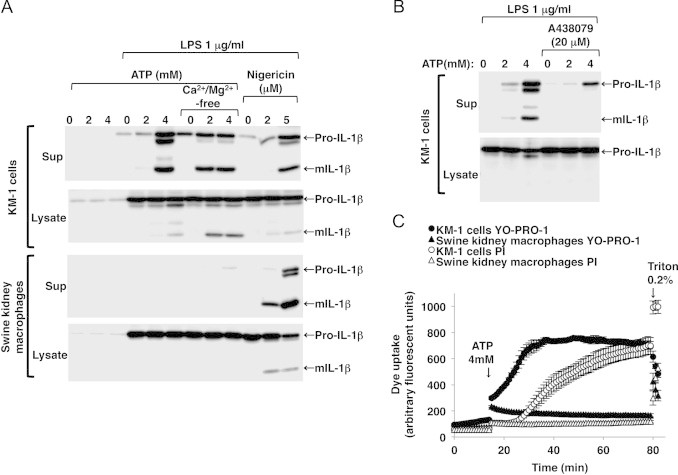
ATP-induced P2X7R-mediated maturation and release of IL-1β and membrane pore formation were not observed in the LPS-primed swine kidney macrophages. mIL-1β release was detected in the LPS-primed mouse KM-1 cells after ATP stimulation (A, sup, first panel). The ATP-induced mIL-1β release was enhanced when the cells were incubated in Ca2+/Mg2+-free buffer (A, sup, first panel), whereas it was inhibited by co-treatment with A438079 (B, sup). ATP-induced mIL-1β release was not detected in the LPS-primed swine kidney macrophages even when they were incubated in Ca2+/Mg2+-free buffer, whereas nigericin, a K+/H+ ionophore, triggered mIL-1β release (A, sup, third panel). Immunoblots are representative of at least three independent experiments. YO-PRO-1 uptake, which preceded PI uptake, was detected in the LPS-primed KM-1 cells after stimulation with 4 mM ATP (C, closed and open circles). Negligible ATP-induced YO-PRO-1 and PI uptake were observed in the LPS-primed swine kidney macrophages (C, closed and open triangles). Maximum dye uptake was estimated after permeabilizing the cells with 0.2% Triton-X100 (C). Fluorescence is expressed in arbitrary units, and the data are shown as mean±SEM values (n=3).
