Abstract
We recently demonstrated in several mammalian species, a novel procedure to obtain liver-macrophages (Kupffer cells) in sufficient numbers and purity using a mixed primary culture of hepatocytes. In this study, we applied this method to the C57BL/6 mouse liver and established an immortalized Kupffer cell line from this mouse strain. The hepatocytes from the C57BL/6 adult mouse liver were isolated by a two-step collagenase perfusion method and cultured in T25 culture flasks. Similar to our previous studies, the mouse hepatocytes progressively changed their morphology into a fibroblastic appearance after a few days of culture. After 7–10 days of culture, Kupffer-like cells, which were contaminants in the hepatocyte fraction at the start of the culture, actively proliferated on the mixed fibroblastic cell sheet. At this stage, a retroviral vector containing the human c-myc oncogene and neomycin resistance gene was introduced into the mixed culture. Gentle shaking of the culture flask, followed by the transfer and brief incubation of the culture supernatant, resulted in a quick and selective adhesion of Kupffer cells to a plastic dish surface. After selection with G418 and cloning by limiting dilutions, a clonal cell line (KUP5) was established. KUP5 cells displayed typical macrophage morphology and were stably passaged at 4–5 days intervals for more than 5 months, with a population doubling time of 19 h. KUP5 cells are immunocytochemically positive for mouse macrophage markers, such as Mac-1, F4/80. KUP5 cells exhibited substantial phagocytosis of polystyrene microbeads and the release of inflammatory cytokines upon lipopolysaccharide stimulation. Taken together, KUP5 cells provide a useful means to study the function of Kupffer cells in vitro.
Keywords: C57BL/6 mouse, Kupffer cells, Immortalization, Mixed hepatocyte culture, Shaking, Attachment
Abbreviations: DAPI, 4′,6-Diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; ELISA, enzyme-linked immunosorbent assay; FC, flow cytometry; GM-CSF, granulocyte-macrophage colony-stimulating factor; RT-PCR, Reverse transcription polymerase chain reaction
1. Introduction
Liver-macrophages known as Kupffer cells are the resident macrophages in the liver [1]. These cells are localized along the sinusoidal space or its immediate vicinity and comprise approximately 10–15% of all liver cells [2]. The main functional roles of Kupffer cells are the phagocytosis of foreign materials, immune surveillance and regulation of hepatic physiological homeostasis [3]. Kupffer cells play a protective role against hepatic damage and promote the regeneration and fibrosis in cholestatic liver injury [1]. However, in pathological conditions, activated Kupffer cells can aggravate liver damage, leading to cirrhosis and eventually failure of the organ. Therefore, Kupffer cells are considered to be an important strategic target for pharmacological intervention against liver disease [4,5].
The methods of isolating Kupffer cells for in vitro studies have been well reported in a variety of mammals, including the mouse [6], rat [7], human [8] and bovine species [9]. However, only a limited number of immortalized Kupffer cell lines have been reported in the mouse [10,11] or Chinese hamster [12]. In our previous studies, we have reported a simple and efficient procedure for obtaining liver-macrophages in a sufficient number and purity using a mixed primary culture of liver cells from rat [13,14], bovine [15] and porcine species [16]. In this study, we applied this method to the adult C57BL/6 mouse liver and established an immortalized Kupffer cell line by a retrovial transduction of c-myc oncogene. The cell line (KUP5) constitutes a useful tool for the in vitro study of Kupffer cells engaged in the innate immune response in liver disease.
2. Materials and methods
2.1. Primary culture of C57BL/6 mouse hepatocytes
The primary culture of adult C57BL/6 male mouse hepatocytes (Hepatocyte Culture Kit; F-4) were purchased from Cosmo Bio. Co., Ltd., Tokyo, Japan. In brief, after a two step perfusion of saline followed by collagenase though the portal vein, hepatocytes were suspended in a growth medium composed of DMEM (D6429, high-glucose type, Sigma-Aldrich, St. Louis, MO) containing 10% heat inactivated FCS (Sanko Junyaku Co. Ltd., Tokyo, Japan) supplemented with 100 µM β-mercaptoethanol (M3148, Sigma-Aldrich), 10 µg/ml insulin (I5500, Sigma-Aldrich), 100 µg/ml streptomycin and 100 U/ml penicillin (15140-122, Life Technologies, Carlsbad, CA), and seeded into tissue culture flasks (surface area: 25 cm2, Sumitomo Bakelite Co., Ltd., Tokyo, Japan) at a density of 1.0×105 cells/cm2. The culture flasks were coated with type I collagen and the culture medium was replaced every 2–3 days. Adult mouse hepatocytes readily attached to the surface of a collagen-coated tissue culture flask and formed a polygonal cobblestone-like monolayer after 2 days of incubation (Fig. 1). As the culture proceeded from days 4 to 7, the hepatocytes lost the epithelial cell morphology and turned into more flattened, fibroblast-like cells (Fig. 1). The morphological transformation process of mouse hepatocytes was very similar to other mammalian species reported previously [13,15,16].
Fig. 1.
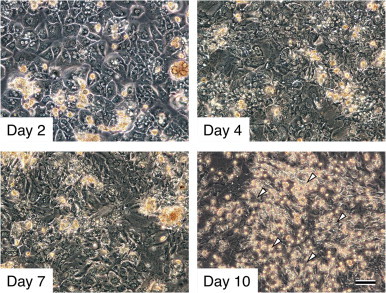
Primary culture of adult C57BL/6 mouse hepatocytes and the proliferation of Kupffer cells. After 2 days of culture, hepatocytes spread onto the surface of the culture flasks and displayed a typical polygonal cobblestone-like morphology. Hepatocytes that lost their epithelial cell morphology after 4 days in culture changed into more flattened, fibroblastic cells. Around days 7–10, phase contrast-bright, round Kupffer-like cells started to proliferate on the fibroblastic cell sheet (arrowheads). The proliferation of Kupffer cells continued and reached a maximum on day 10 and continued thereafter. Scale bar = 100 µm.
2.2. Infection with a retrovial vector containing c-myc and isolation of immortalized Kupffer cells
After approximately 10 days of culture, when most of the hepatocytes had transformed into fibroblastic cells, round macrophage-like cells started to proliferate vigorously on the cell sheet and form distinctive colonies (Fig. 1), as reported previously [13,15,16]. These macrophage-like cells probably originated from macrophages, which were contaminants in the hepatocyte fraction at the start of the culture [13]. After shaking the culture flasks, macrophage-like cells were obtained by the selective adhesion to non-tissue culture grade plastic dishes [13], and used as the primary Kupffer cells. Also, at this stage, a retroviral vector containing human c-myc oncogene and the neomycin resistance gene (a gift from Dr. M. Noda, Kyoto University, Japan) was introduced into the mixed culture. After infection for three consecutive days (Fig. 2A), the loosely attached liver-macrophages were then suspended by reciprocal shaking of the culture flasks at 180 strokes per minute for 20 min at 37 °C. The culture medium was transferred into 60 mm non-tissue culture grade plastic dishes (351,007, Corning). After incubation for 30 min at 37 °C, followed by rinsing with PBS, the liver-macrophages attached onto the dish surface (Fig. 2B) were subjected to selection with G418 disulfate (16512-52, Nacalai Tesque Inc., Kyoto, Japan) at 600 µg/ml of the growth medium. G418-resistant liver-macrophages were harvested by scraping and pipeting and subcultured into new 60 mm non-tissue culture grade plastic dishes. After expansion, these cells were suspended in a cell freezing medium (Cell Banker, CB011, Takara Bio, Inc., Shiga, Japan), aliquoted in cell freezing vials (MS-4503, Sumitomo Bakelite Co., Ltd.) and kept frozen in liquid nitrogen. These cells were cloned twice by a limiting dilution in a 96-well plate, and a representative clone (KUP5) was established and characterized. For the growth analysis of KUP5, the cells were seeded in 60 mm non-tissue culture grade plastic dishes (5×104 cells/dish in duplicate). After 4–5 days of culture, the cells were harvested and the cell number in the dish counted by a disposable hemocytometer. An aliquot of the cells (5×104 cells) was seeded into new 60 mm non-tissue culture grade plastic dishes to continue the passage. Population doubling during the culture period was calculated and the cumulative number of doublings plotted against the cumulative culture days (Fig. 2C).
Fig. 2.
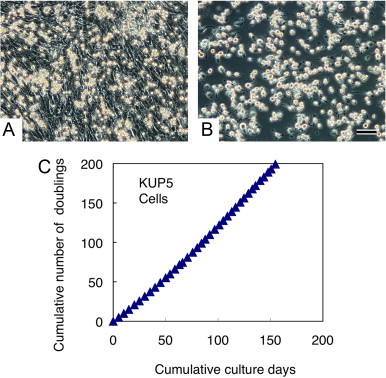
Selective isolation of immortalized C57BL/6 mouse Kupffer cells by a shaking and attachment method. Kupffer cells that proliferated on the mixed cell sheet (A) were infected with a retroviral vector containing the human c-myc oncogene and neomycin resistance gene. After infection for 3 days, Kupffer cells were suspended into the culture medium by gently shaking the flasks, subsequently transferred into a non-tissue culture grade plastic dish and incubated for 30 min at 37 °C. Kupffer cells promptly attached to the dish surface, while other, contaminating fibroblastic cells remained suspended. After a rinse with PBS, a highly purified Kupffer cell population was obtained (B). After selection and cloning with neomycin containing medium, the KUP5 cell line was established. The stable proliferative capacity of KUP5 cells was demonstrated by the continuous passage at 4–5 days intervals for 5 months with a constant population doubling time of approximately 19 h (C). Scale bar = 100 µm.
2.3. Immunocytochemistry
The primary Kupffer cells and KUP5 cells were seeded on eight-well chamber glass slides (354,118, Corning) at the density of 2×104 cells/well with the growth medium. The next day, the cells were washed with PBS, fixed with 95% ethanol and 1% acetic acid, and processed for immunocytochemistry, as described [17]. For comparison, immortalized macrophage cell lines established from C57BL/6 mouse by the same c-myc-containing retroviral vector were examined in parallel. MG6 is a microglial cell line [18,19] and BMDM is a bone marrow-derived macrophage cell line [20,21]. The primary antibodies were as follows: rat monoclonal anti-mouse CD11b (Mac-1; Bio-Legend); rat monoclonal anti-mouse F4/80 (Bio-Legend); rat IgG (Sigma-Aldrich) as a negative control. After rinsing the slides with PBS containing 0.05% Tween 20, HRP-conjugated goat anti-rat IgG (Life Technologies) for the rat primary antibodies was used to visualize the antibody-antigen reaction. The immunostained slides were observed and photographed with a Leica DM5000B phase contrast microscope equipped with a digital camera system.
2.4. Phagocytic assay
Fluorescence-labeled polystyrene microbeads (1.0 µm diameter, #17154, Polysciences, Inc., Warrington, PA) were diluted to 1:800 in the growth medium and added to the primary Kupffer cells, KUP5, MG6 and BMDM cells seeded in 60 mm non-tissue culture grade plastic dishes (5×105 cells/plate). After 1 and 2 h, the cells in the plastic dishes were rinsed with PBS three times to remove nonphagocytosed beads [22,23], harvested with TrypLE Express and fixed with 3.7% formalin in PBS at room temperature for 15 min. After washing with PBS, cells were suspended in 0.5 ml of Iso Flow (Beckman Coulter, Fullerton, CA) and analyzed with a flow cytometer (Epics XL-MCL, Beckman Coulter) for phagocytosis of the fluorescence-labeled microbeads.
2.5. Cytokine production
The primary Kupffer cells, KUP5, MG6 and BMDM cells were seeded in a 12-well cell culture plate at a density of 2×105 cells/well. The next day, the medium was replaced by growth medium containing lipopolysaccharide (L3129, Sigma-Aldrich) at 0.1–1.0 µg/ml. After incubation for 24 h at 37 °C, the culture supernatant was collected, filtered with a membrane filter (0.20 µm pore size, Millipore Millex) and stored at −80 °C until use. Aliquots of the samples were assayed using mouse cytokine ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacture's instructions. The experiments were independently performed three times and the cytokine concentrations in the culture supernatant are expressed as the mean value±SEM.
2.6. Induction of multinucleated giant cells by GM-CSF
The KUP5 and MG6 cells were seeded in eight-well chamber glass slides at the density of 2×104 cells/well along with the growth medium containing recombinant mouse GM-CSF (415-ML, R&D Systems, Minneapolis, MN) at 10 ng/ml. After incubation for 3 days, the cells were washed with PBS, fixed with 95% ethanol and 1% acetic acid. After rinsing with PBS, the slides were mounted with mounting medium containing DAPI and photographed with a Leica DM5000B fluorescent microscope system equipped with a digital camera.
3. Results and discussion
3.1. Morphological and immunocytochemical characterization of KUP5 cells
We established an immortalized Kupffer cell line (KUP5) from a mixed primary culture of adult C57BL/6 mouse hepatocytes by a retrovial transduction of the c-myc oncogene. These macrophage-like cells probably have originated from Kupffer cells, which were contaminants in the parenchymal hepatocyte fraction at the start of the culture. These cells proliferated vigorously in response to the specific culture environment provided by the morphologically transformed mouse hepatocytes [13], and thus were susceptible for the infection by the retroviral vector.
KUP5 cells had a round ameboid cell body with filopodia and lamellipodia, displaying a typical macrophage morphology in these culture conditions (Fig. 2B). KUP5 cells exhibited a stable proliferative capacity, as demonstrated by the successful passage at 4–5 days intervals for more than 5 months with a constant population doubling time of approximately 19 h (Fig. 2C). The expression of human c-myc oncogene was confirmed by RT-PCR analysis in KUP5 cells (data not shown). In addition, KUP5 cells can be frozen in a conventional cell-freezing medium. After cryopreservation in liquid nitrogen for 3 years, followed by thawing, KUP5 cells still retained its morphological properties and stable proliferative capacity (data not shown).
The primary Kupffer cells, KUP5, MG6 and BMDM cells were strongly positive for mouse macrophage markers, such as Mac-1 and F4/80 (Fig. 3). Mac-1 and F4/80 are cell surface markers, but the antibody–antigen reaction was so intense that the cellular and nuclear structures were difficult to distinguish under a phase contrast microscope. A negative control, rat IgG showed only faint immunostaining (Fig. 3). In addition, multinucleated giant cells were occasionally observed in the KUP5 culture (Fig. 3, arrowheads), suggesting that these cells tend to fuse with each other during culture. Multinucleated giant cell formation is associated with the inflammation associated with macrophages [24–26], including Kupffer cells [27]. These results suggest that KUP5 cells have the typical biological properties of macrophages, i. e. Kupffer cells or their precursors, and proliferated in the mixed primary culture of mouse liver cells.
Fig. 3.
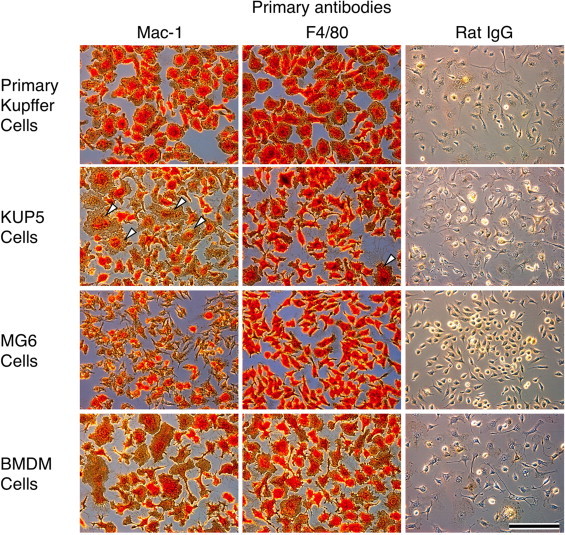
Immunocytochemical staining of the primary Kupffer cells, KUP5, MG6 and BMDM cell plated on eight-well chamber glass slides in DMEM-based medium containing 10% FCS. The cells were fixed and stained with specific antibodies against Mac-1, F4/80, and rat IgG, as described in Section 2. Mac-1 and F4/80 are cell surface markers, but the antibody–antigen reaction was so intense that the cellular and nuclear structures were difficult to distinguish under a phase contrast microscope. A negative control, rat IgG showed only faint immunostaining. Multinucleated giant cells were occasionally observed in the culture of KUP5 cells (arrowheads). Scale bar = 100 µm.
Using the same retroviral vector, we previously established microglial cell lines from a C57BL/6 mouse strain (MG6, deposited at RIKEN, Cell Bank, RCB2403) [18], as well as transgenic and gene-knockout mouse strains [28,29]. In addition, this retroviral vector has a capacity for infectivity of mouse bone marrow-derived macrophages in culture [20], suggesting that the present retroviral vector is a powerful tool for immortalizing mouse macrophages. Bioassay revealed that there is no infectious viral particles produced in the culture supernatant of KUP5 cells or other microglial and macrophage cell lines immortalized by the same retroviral vector (data not shown).
3.2. Phagocytic activity
The primary Kupffer cells, KUP5, MG6 and BMDM cells phagocytosed polystyrene microbeads. The phagocytic activities of the cells were quantitatively demonstrated by FC (Fig. 4), indicating that the proportion of the fluorescence-positive cells increased to more than 95% after 2 h of administration. The levels of phagocytosis did not differ among these cell types. These results demonstrate the substantial phagocytic activity of KUP5 cells, which is a distinctive characteristic of macrophages in the liver [22,23,30].
Fig. 4.
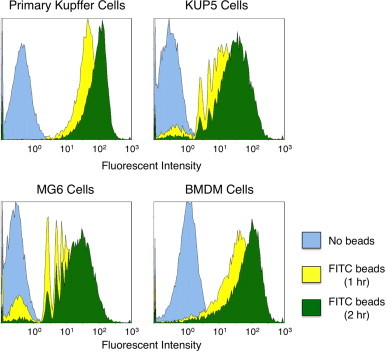
Phagocytosis of fluorescence-labeled microbeads by the primary Kupffer cells, KUP5, MG6 and BMDM cells. After incubation for 1 or 2 h at 37 °C in the presence of microbeads, cells were washed three times with PBS, fixed with formalin and analyzed by FC, as described in Section 2.
3.3. ELISA analysis of cytokine release
We assayed the capacity of the primary Kupffer cells, KUP5, MG6 and BMDM cells to produce inflammatory and anti-inflammatory cytokines in response to lipopolysaccharide. All of these cells secreted a substantial amount inflammatory cytokines, such as TNFα, IL-6 and RANTES (Fig. 5a). These results suggest that these cells primarily have strong M1 phenotype in the response to bacterial endotoxin [31]. The primary Kupffer cells and KUP5 cells secreted a substantial amount of anti-inflammatory cytokine (IL-10) after stimulation with lipopolysaccharide for 24 h (Fig. 5b). This is in accordance to the previous reports on the response of Kupffer cells to lipopolysaccharide [32,33]. In sharp contrast, MG6 and BMDM did not secrete measurable IL-10 (Fig. 5b). The capability to secrete IL-10 upon lipopolysaccharide stimulation may suggest the inherent property of the primary Kuppfer cells might be maintained in its immortalized KUP5 cells. Further studies are needed to compare the anti-inflammatory response profiles of KUP5, MG6 and BMDM cells to various stimuli in detail.
Fig. 5.
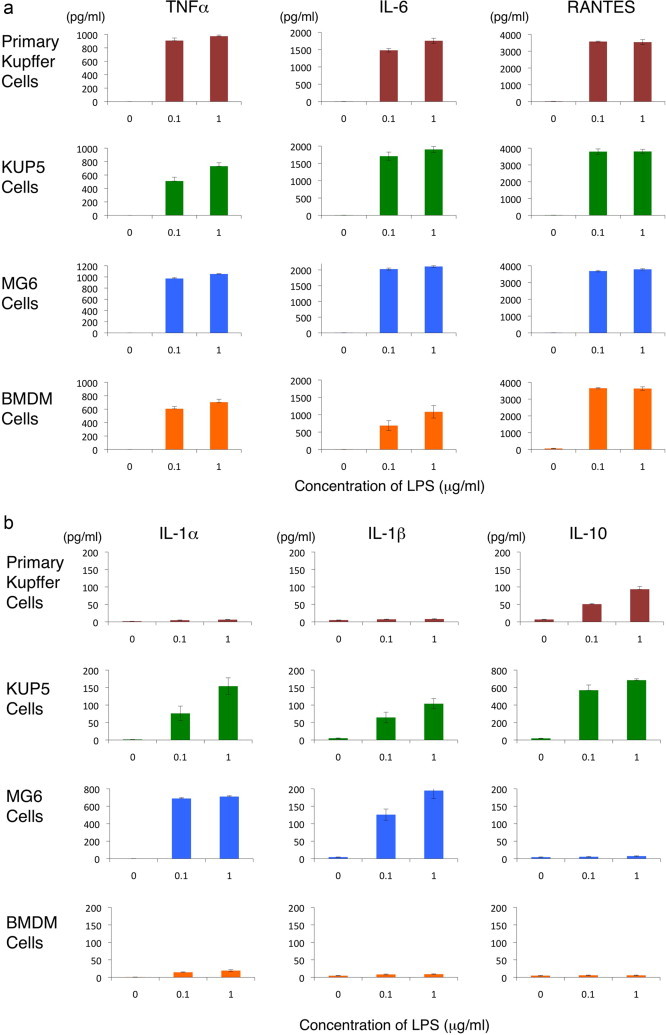
Secretion of inflammatory and anti-inflammatory cytokines from the primary Kupffer cells, KUP5, MG6 and BMDM cells after stimulation with lipopolysaccharide for 24 h. The experiments were independently performed three times. The cytokine concentrations in the culture supernatant were quantified using specific mouse ELISA kits and expressed as the mean value±SEM.
The primary Kupffer cells did not secrete measurable IL-1α and IL-1β and KUP5 cells secreted only low levels of these cytokine (ca. 50–150 pg/ml) after stimulation with lipopolysaccharide (Fig. 5b). MG6 cells secrete slightly higher levels of IL-1α and IL-1β (ca. 200–600 pg/ml) BMDM cells did not secrete these cytokines (Fig. 5b). Although there were slight differences among the cells, this is in agreement with macrophages of human or murine origin, which usually require a second stimulus, such as ATP, for the maturation and release of these cytokines [34,35]. In relation to this scenario, some of the KUP5 and MG6 cells showed morphological signs of cell death (phase-contrast dark floating cells) after 24 h of lipopolysaccharide treatment. Intracellular ATP may have been released from dead KUP and MG6 cells, and stimulated the maturation and release of IL-1α and IL-1β in an autocrine fashion. The cellular sensitivity of KUP5 and MG6 to lipopolysaccharide may not simply be explained by the introduction of c-myc oncogene alone, as BMDM cells seems to be more tolerant. In untreated negative controls, the concentrations of all the cytokines were under the detection limit of the ELISA kits.
3.4. Multinucleated giant cell formation induced by recombinant mouse GM-CSF
Multinucleated giant cell formation is suggested to associate with the inflammation of macrophages [24–27] and the fusion process is enhanced by specific cytokines, such as GM-CSF [36,37]. In accordance with these reports, recombinant mouse GM-CSF induced multinucleated giant cell formation in KUP5 cells, but not in MG6 cells (Fig. 6). In contrast, only a small number of multinucleated giant cells were observed in untreated controls (Fig. 6). For a negative control, recombinant human GM-CSF at the same concentration showed no effect on the increase of KUP5 or MG6 cell fusion (data not shown), indicating the species specificity of this recombinant cytokine. The GM-CSF-induced fusion of KUP5 cells may provide a useful system for analyzing the cellular and molecular mechanisms of macrophage fusion [26,38].
Fig. 6.
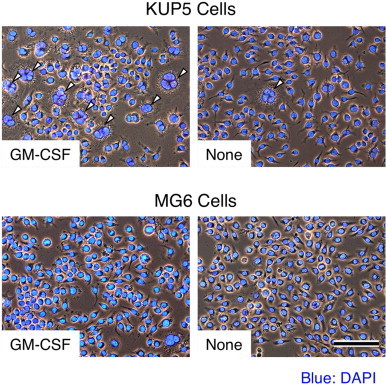
Induction of cell fusion in KUP5 cells by GM-CSF. Recombinant mouse GM-CSF at 10 ng/ml induced multinucleated giant cell formation in KUP5 cells (arrowheads), whereas only a very few multinucleated giant cells were observed in untreated controls (arrowhead). In contrast, recombinant mouse GM-CSF did not induce multinucleated giant cell formation in MG6 cells. The cells were fixed and mounted with DAPI-containing mounting medium. Photomicrographs were taken under phase contrast/fluorescence microscopy, as described in Section 2. Scale bar = 100 µm.
3.5. Conclusion
KUP5, a c-myc-immortalized C57BL/6 mouse Kupffer cell line, constitutes a useful tool for the in vitro study of the innate immune responses of these specific cells during various hepatic diseases such as non-alcoholic fatty liver disease [39] or nonalcoholic steatohepatitis [40]. In addition, we recently examined the purinergic P2X and P2Y signalings of KUP5 cells on the production of IL-1β [41] and IL-6 [42], suggesting KUP5 cell line provides a good platform for both drug discovery and basic scientific study. We plan to deposit this cell line in RIKEN, Cell Bank for public access.
Acknowledgments
This study was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomic-based Technology for Agricultural Improvement, AGB-1004) and the NIAS Strategic Research Fund from National Institute of Agrobiological Sciences.
References
- 1.Crispe I.N. The liver as a lymphoid organ. Annual Review of Immunology. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. 19302037 [DOI] [PubMed] [Google Scholar]
- 2.Hendriks H.F., Brouwer A., Knook D.L. Isolation, purification, and characterization of liver cell types. Methods in Enzymology. 1990;190:49–58. doi: 10.1016/0076-6879(90)90008-o. 1965003 [DOI] [PubMed] [Google Scholar]
- 3.Bilzer M., Roggel F., Gerbes A.L. Role of Kupffer cells in host defense and liver disease. Liver International: Official Journal of the International Association for the Study of the Liver. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. 17105582 [DOI] [PubMed] [Google Scholar]
- 4.Heymann F., Trautwein C., Tacke F. Monocytes and macrophages as cellular targets in liver fibrosis. Inflammation and Allergy Drug Targets. 2009;8:307–318. doi: 10.2174/187152809789352230. 19534673 [DOI] [PubMed] [Google Scholar]
- 5.Roberts R.A., Ganey P.E., Ju C., Kamendulis L.M., Rusyn I., Klaunig J.E. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicological Sciences: an Official Journal of the Society of Toxicology. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. 17122412 [DOI] [PubMed] [Google Scholar]
- 6.Janousek J., Strmen E., Gervais F. Purification of murine Kupffer cells by centrifugal elutriation. Journal of Immunological Methods. 1993;164:109–117. doi: 10.1016/0022-1759(93)90281-b. 8360500 [DOI] [PubMed] [Google Scholar]
- 7.Olynyk J.K., Clarke S.L. Isolation and primary culture of rat Kupffer cells. Journal of Gastroenterology and Hepatology. 1998;13:842–845. doi: 10.1111/j.1440-1746.1998.tb00746.x. 9736181 [DOI] [PubMed] [Google Scholar]
- 8.Alabraba E.B., Curbishley S.M., Lai W.K., Wigmore S.J., Adams D.H., Afford S.C. A new approach to isolation and culture of human Kupffer cells. Journal of Immunological Methods. 2007;326:139–144. doi: 10.1016/j.jim.2007.06.014. 17692868 [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka M., Nakajima Y., Ito T., Mikami O., Tanaka S., Miyazaki S. Primary culture and expression of cytokine mRNAs by lipopolysaccharide in bovine Kupffer cells. Veterinary Immunology and Immunopathology. 1997;58:155–163. doi: 10.1016/s0165-2427(97)00030-5. 9336883 [DOI] [PubMed] [Google Scholar]
- 10.Peng Y., Murr M.M. Establishment of immortalized rat Kupffer cell lines. Cytokine. 2007;37:185–191. doi: 10.1016/j.cyto.2007.03.003. 17502155 [DOI] [PubMed] [Google Scholar]
- 11.Dory D., Echchannaoui H., Letiembre M., Ferracin F., Pieters J., Adachi Y. Generation and functional characterization of a clonal murine periportal Kupffer cell line from H-2Kb -tsA58 mice. Journal of Leukocyte Biology. 2003;74:49–59. doi: 10.1189/jlb.0302133. 12832442 [DOI] [PubMed] [Google Scholar]
- 12.Clark J.M., Pateman J.A. A study of cultured fetal and SV40-transformed Chinese hamster Kupffer cells. Experimental Cell Research. 1978;114:47–56. doi: 10.1016/0014-4827(78)90034-4. 207542 [DOI] [PubMed] [Google Scholar]
- 13.Kitani H., Takenouchi T., Sato M., Yoshioka M., Yamanaka N. A novel isolation method for macrophage-like cells from mixed primary cultures of adult rat liver cells. Journal of Immunological Methods. 2010;360:47–55. doi: 10.1016/j.jim.2010.06.004. 20600081 [DOI] [PubMed] [Google Scholar]
- 14.Kitani H., Takenouchi T., Sato M., Yoshioka M., Yamanaka N. A simple and efficient method to isolate macrophages from mixed primary cultures of adult liver cells. Journal of Visualized Experiments. 2011 doi: 10.3791/2757. 21654622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitani H., Yoshioka M., Takenouchi T., Sato M., Yamanaka N. Isolation and characterization of macrophages from a mixed primary culture of bovine liver cells. Veterinary Immunology and Immunopathology. 2011;140:341–345. doi: 10.1016/j.vetimm.2011.01.011. 21334751 [DOI] [PubMed] [Google Scholar]
- 16.Kitani H., Yoshioka M., Takenouchi T., Sato M., Yamanaka N. Characterization of the liver-macrophages isolated from a mixed primary culture of neonatal swine hepatocytes. Results in Immunology. 2014;4:1–7. doi: 10.1016/j.rinim.2014.01.001. 24707456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takenouchi T., Iwamaru Y., Sato M., Yokoyama T., Shinagawa M., Kitani H. Establishment and characterization of SV40 large T antigen-immortalized cell lines derived from fetal bovine brain tissues after prolonged cryopreservation. Cell Biology International. 2007;31:57–64. doi: 10.1016/j.cellbi.2006.09.006. 17049468 [DOI] [PubMed] [Google Scholar]
- 18.Takenouchi T., Ogihara K., Sato M., Kitani H. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochimica et Biophysica Acta. 2005;1726:177–186. doi: 10.1016/j.bbagen.2005.08.001. 16122875 [DOI] [PubMed] [Google Scholar]
- 19.Nakamichi K., Saiki M., Kitani H., Kuboyama Y., Morimoto K., Takayama-Ito M. Suppressive effect of simvastatin on interferon-beta-induced expression of CC chemokine ligand 5 in microglia. Neuroscience Letters. 2006;407:205–210. doi: 10.1016/j.neulet.2006.08.044. 16978784 [DOI] [PubMed] [Google Scholar]
- 20.Sakuma C., Sato M., Takenouchi T., Chiba J., Kitani H. Critical roles of the WASP N-terminal domain and Btk in LPS-induced inflammatory response in macrophages. PloS One. 2012;7:e30351. doi: 10.1371/journal.pone.0030351. 22253930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuma C., Sato M., Takenouchi T., Chiba J., Kitani H. Single-chain variable fragment intrabody impairs LPS-induced inflammatory responses by interfering with the interaction between the WASP N-terminal domain and Btk in macrophages. Biochemical and Biophysical Research Communications. 2012;423:164–169. doi: 10.1016/j.bbrc.2012.05.105. 22634306 [DOI] [PubMed] [Google Scholar]
- 22.Itoh Y., Okanoue T., Morimoto M., Nagao Y., Mori T., Hori N. Functional heterogeneity of rat liver macrophages: Interleukin-1 secretion and Ia antigen expression in contrast with phagocytic activity. Liver. 1992;12:26–33. doi: 10.1111/j.1600-0676.1992.tb00551.x. 1564982 [DOI] [PubMed] [Google Scholar]
- 23.Olynyk J.K., Britton R.S., Stephenson A.H., Leicester K.L., O’Neill R., Bacon B.R. An in vitro model for the study of phagocytosis of damaged hepatocytes by rat Kupffer cells. Liver. 1999;19:418–422. doi: 10.1111/j.1478-3231.1999.tb00071.x. 10533800 [DOI] [PubMed] [Google Scholar]
- 24.Lemaire I., Falzoni S., Leduc N., Zhang B., Pellegatti P., Adinolfi E. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. Journal of Immunology (Baltimore, Md.: 1950) 2006;177:7257–7265. doi: 10.4049/jimmunol.177.10.7257. 17082644 [DOI] [PubMed] [Google Scholar]
- 25.McNally A.K., Anderson J.M. Macrophage fusion and multinucleated giant cells of inflammation. Advances in Experimental Medicine and Biology. 2011;713:97–111. doi: 10.1007/978-94-007-0763-4_7. 21432016 [DOI] [PubMed] [Google Scholar]
- 26.Lemaire I., Falzoni S., Adinolfi E. Purinergic signaling in giant cell formation. Frontiers in Bioscience (Elite Edition) 2012;4:41–55. doi: 10.2741/359. 22201854 [DOI] [PubMed] [Google Scholar]
- 27.Pulford K., Souhami R.L. Cell division and giant cell formation in Kupffer cell cultures. Clinical and Experimental Immunology. 1980;42:67–76. 7460393 [PMC free article] [PubMed] [Google Scholar]
- 28.Sato M., Ogihara K., Sawahata R., Sekikawa K., Kitani H. Impaired LPS-induced signaling in microglia overexpressing the Wiskott–Aldrich syndrome protein N-terminal domain. International Immunology. 2007;19:901–911. doi: 10.1093/intimm/dxm074. 17698982 [DOI] [PubMed] [Google Scholar]
- 29.Iwamaru Y., Takenouchi T., Ogihara K., Hoshino M., Takata M., Imamura M. Microglial cell line established from prion protein-overexpressing mice is susceptible to various murine prion strains. Journal of Virology. 2007;81:1524–1527. doi: 10.1128/JVI.01379-06. 17121794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caperna T.J., Failla M.L., Kornegay E.T., Richards M.P., Steele N.C. Isolation and culture of parenchymal and nonparenchymal cells from neonatal swine liver. Journal of Animal Science. 1985;61:1576–1586. doi: 10.2527/jas1985.6161576x. 4086406 [DOI] [PubMed] [Google Scholar]
- 31.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nature Reviews: Immunology. 2011;11:723–737. doi: 10.1038/nri3073. 21997792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knolle P., Schlaak J., Uhrig A., Kempf P., Meyer zum Büschenfelde K.H., Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. Journal of Hepatology. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. 7790711 [DOI] [PubMed] [Google Scholar]
- 33.Valatas V., Kolios G., Manousou P., Xidakis C., Notas G., Ljumovic D. Secretion of inflammatory mediators by isolated rat Kupffer cells: The effect of octreotide. Regulatory Peptides. 2004;120:215–225. doi: 10.1016/j.regpep.2004.03.009. 15177940 [DOI] [PubMed] [Google Scholar]
- 34.Dinarello C.A. The IL-1 family and inflammatory diseases. Clinical and Experimental Rheumatology. 2002;20:S1–13. 14989423 [PubMed] [Google Scholar]
- 35.Takenouchi T., Sugama S., Iwamaru Y., Hashimoto M., Kitani H. Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Critical Reviews in Immunology. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. 19673687 [DOI] [PubMed] [Google Scholar]
- 36.Lemaire I., Yang H., Lauzon W., Gendron N. M-CSF and GM-CSF promote alveolar macrophage differentiation into multinucleated giant cells with distinct phenotypes. Journal of Leukocyte Biology. 1996;60:509–518. doi: 10.1002/jlb.60.4.509. 8864136 [DOI] [PubMed] [Google Scholar]
- 37.Yoshihara K., Nagata R., Muneta Y., Inumaru S., Yokomizo Y., Mori Y. Generation of multinucleated giant cells in vitro from bovine monocytes and macrophages. Journal of Veterinary Medical Science/the Japanese Society of Veterinary Science. 2004;66:1065–1069. doi: 10.1292/jvms.66.1065. 15472469 [DOI] [PubMed] [Google Scholar]
- 38.Helming L., Gordon S. Molecular mediators of macrophage fusion. Trends in Cell Biology. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. 19733078 [DOI] [PubMed] [Google Scholar]
- 39.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: The emerging view. Journal of Hepatology. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. 19447517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imajo K., Fujita K., Yoneda M., Nozaki Y., Ogawa Y., Shinohara Y. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metabolism. 2012;16:44–54. doi: 10.1016/j.cmet.2012.05.012. 22768838 [DOI] [PubMed] [Google Scholar]
- 41.Kojima S., Negishi Y., Tsukimoto M., Takenouchi T., Kitani H., Takeda K. Purinergic signaling via P2X7 receptor mediates IL-1β production in Kupffer cells exposed to silica nanoparticle. Toxicology. 2014;321:13–20. doi: 10.1016/j.tox.2014.03.008. 24685903 [DOI] [PubMed] [Google Scholar]
- 42.Ishimaru M., Yusuke N., Tsukimoto M., Harada H., Takenouchi T., Kitani H. Purinergic signaling via P2Y receptors up-mediates IL-6 production by liver macrophages/Kupffer cells. Journal of Toxicological Sciences. 2014;39:413–423. doi: 10.2131/jts.39.413. 24849676 [DOI] [PubMed] [Google Scholar]


