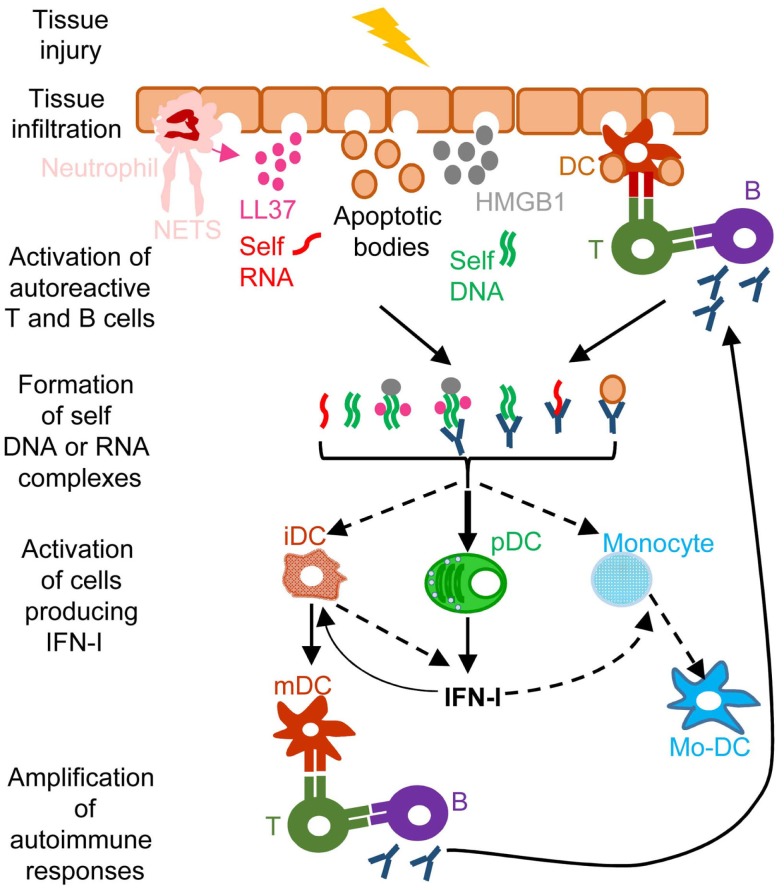
Figure 4.
A simplified model of the deleterious role of IFN-I in several autoimmune diseases. When exposed to different kinds of injuries (microbial infection, commensal microbiota dysbiosis, chemical or physical insults), healthy tissues can undergo cell damage and death. These events induce the release of apoptotic bodies encompassing self RNA or DNA. Neutrophil recruitment and activation in inflamed tissues can also constitute a potent source of self nucleic acids, through the release of neutrophil extracellular traps (NET). Self RNA or DNA can associate with cationic peptides (e.g., LL37) as shown in psoriatic patients or with inflammatory molecules (e.g., high mobility group box 1, HMGB1) to generate nanoparticles that are extremely efficient for IFN-I production by pDC and eventually other cell types. pDC can also be efficiently activated for IFN-I production by immune complexes (ICs) generated by the association between self nucleic acids and auto-antibodies as frequently found in the serum of systemic lupus erythematosus patients. IFN-I promote the differentiation and/or the maturation of antigen-presenting cells, in particular different subsets of DC. Activated DC can then present self-antigens for activation of auto-reactive T CD4+ cells, including follicular helper lymphocytes, which in turn activate auto-reactive B cells for auto-antibody secretion, leading to a vicious circle of reciprocal activation between innate and auto-reactive adaptive immune cells. iDC, immature DC; mDC, mature DC; Mo-DC, monocyte-derived DC. See main text for further details.