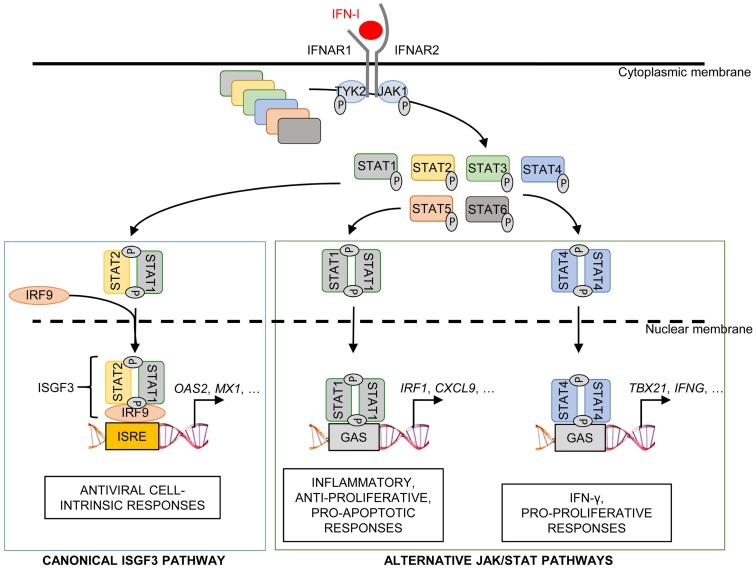
Figure 8.
Schematic representation of the ISGF3 and alternative JAK/STAT signaling pathways induced by IFN-I. The receptor for IFN-I, IFNAR, is composed of two chains, IFNAR1 and IFNAR2, which are respectively associated with the JAK family kinases TYK2 and JAK1. IFN-I binding to IFNAR triggers the phosphorylation of TYK2 and JAK1, which in turn phosphorylate a variety of STAT proteins. Activated STATs are able to form complexes, as homo- or hetero-dimers. The heterodimer STAT1-STAT2 binds to a third partner, IFN-regulatory factor 9 (IRF9), in order to form the ISGF3 complex. This complex translocates into the nucleus and binds to specific regulatory sequences, IFN-stimulated response elements (ISRE), to activate the expression of many interferon-stimulated genes (ISGs). In particular, ISGF3 induces most, if not all, of the ISGs encoding effector molecules of cell-intrinsic anti-viral defenses such as OAS or MX1. Alternative JAK/STAT pathways include the formation of STAT1 or STAT4 homodimers, which may drive different functional responses to IFN-I. STAT1 homodimers bind to IFNγ-activated sequences (GAS) in the promoter of certain ISGs, which may promote inflammatory, anti-proliferative, and pro-apoptotic responses. STAT4 homodimers also bind to GAS but promote IFN-γ production and pro-proliferative responses.