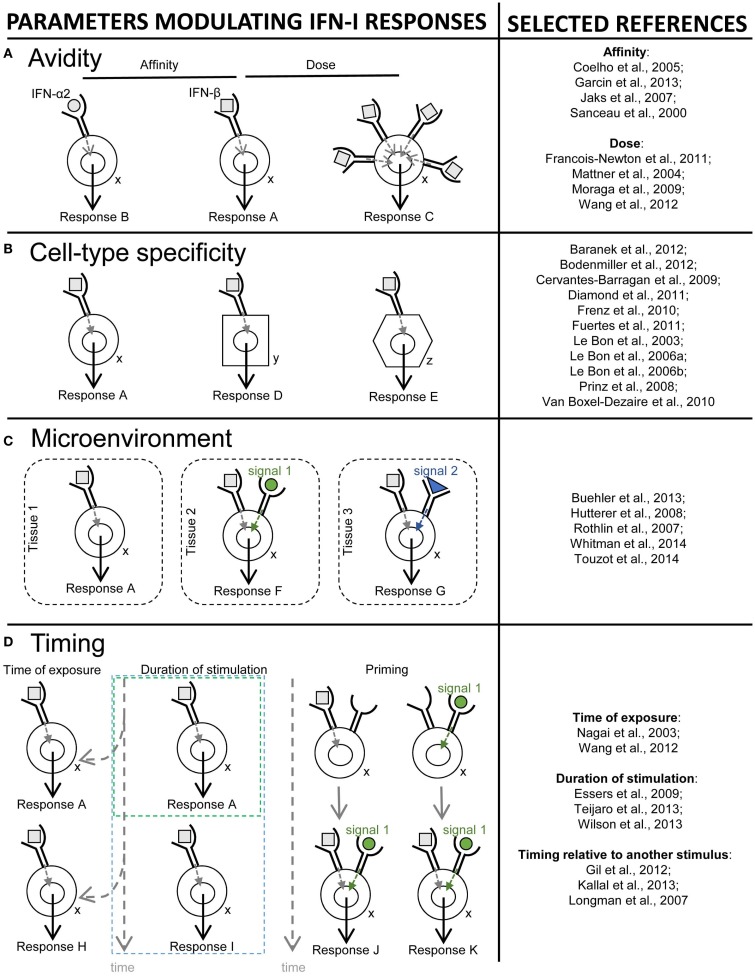
Figure 9.
Schematic illustration of different mechanisms controlling the diversity of IFN-I effects. Different parameters that contribute to promote and control the diversity of IFN-I responses are depicted on the left side of the figure. References of papers illustrating each mechanism are given on the right side of the figure. (A) Avidity (a combination of affinity and dose). For example, the affinity of IFN-β for IFNAR1 is 100-times higher than that of IFN-α2, and IFNβ is much more potent in inhibiting cellular proliferation or monocyte differentiation into osteoclasts (response A), while both IFN-I subtypes are equipotent in establishing an anti-viral state (response B). The same subset of IFN-I can also exert different biological effects at low versus high doses. For example, low, but not high, doses of IFN-β protect BALB/c mice from progressive cutaneous and fatal visceral disease after Leishmania major infection. (B) Cell type specificity. Mouse DC but not NK cells are strong responders to IFN-I, and cell-intrinsic responses to IFN-I are critical in DC but not in some other cell types for immune defenses against viral infections or tumors. (C) Tissue microenvironment. The response of a given cell type to a given dose of a specific subset of IFN-I can also be modulated by the microenvironment of the cell. For example, in cancer, protective IFN-I effects on infiltrating DC or other immune cells might be dampened by inhibitors of IFNAR signaling locally produced by the tumor, such as ligands of the TAM receptor tyrosine kinases. (D) Timing. Differences in the time and duration of exposure to IFN-I can also determine distinct functional outcomes. For example, during viral infections, early and transient high levels of IFN-I promote protective DC and T cell responses, while delayed, chronic and low level IFN-I production compromises host immune defenses and promotes chronic viral infections. Within a given cell type, the outcome of IFN-I stimulation also depends on time of exposure to these cytokines relative to other modulatory signals (timing relative to other stimuli). For example, in naïve CD8 T cells, TCR signaling prior to IFN-I stimulation leads to increased expression of STAT4 and promotes IFN-γ production and proliferation, while IFN-I stimulation prior to TCR triggering leads to STAT1-dependent anti-proliferative and pro-apoptotic effects.