Abstract
We previously showed that the transcription factor Mafb is essential for podocyte differentiation and foot process formation. Podocytes are susceptible to injury in diabetes, and this injury leads to progression of diabetic nephropathy. In this study, we generated transgenic mice that overexpress Mafb in podocytes using the nephrin promoter/enhancer. To examine a potential pathogenetic role for Mafb in diabetic nephropathy, Mafb transgenic mice were treated with either streptozotocin or saline solution. Diabetic nephropathy was assessed by renal histology and biochemical analyses of urine and serum. Podocyte-specific overexpression of Mafb had no effect on body weight or blood glucose levels in either diabetic or control mice. Notably, albuminuria and changes in BUN levels and renal histology observed in diabetic wild-type animals were ameliorated in diabetic Mafb transgenic mice. Moreover, hyperglycemia-induced downregulation of Nephrin was mitigated in diabetic Mafb transgenic mice, and reporter assay results suggested that Mafb regulates Nephrin directly. Mafb transgenic glomeruli also overexpressed glutathione peroxidase, an antioxidative stress enzyme, and levels of the oxidative stress marker 8-hydroxydeoxyguanosine decreased in the urine of diabetic Mafb transgenic mice. Finally, Notch2 expression increased in diabetic glomeruli, and this effect was enhanced in diabetic Mafb transgenic glomeruli. These data indicate Mafb has a protective role in diabetic nephropathy through regulation of slit diaphragm proteins, antioxidative enzymes, and Notch pathways in podocytes and suggest that Mafb could be a therapeutic target.
Diabetic nephropathy is the major cause of ESRD leading to dialysis. The earliest manifestations of the disease occur in the kidney glomerulus, and consequently, most attention to mechanisms has focused on altered responses of diabetic glomerular cells, especially mesangial cells and more recently, podocytes.1 Podocytes are terminally differentiated epithelial cells that are key components of the selective permeability barrier of the glomerular basement membrane.2 Podocytes seem to be quite susceptible to injury in diabetes,3 and this injury leads to loss of podocytes, in part, by apoptosis.4,5 This podocyte depletion seems to be a relatively early event in the evolution of diabetic nephropathy and predicts clinical progression of the disease.6 The factors that lead to podocyte loss in diabetes are being actively investigated and include enhanced oxidative stress5 and activation of the renin-angiotensin-aldosterone system.7 However, little is known about the specific mechanism.
Mafb is a basic leucine zipper transcription factor belonging to the large Maf family.8 Maf family proteins share a conserved basic region and leucine zipper motif that mediates dimer formation and DNA binding to the Maf recognition element (MARE).9 Analysis of Mafb-deficient mice has revealed that Mafb is essential for podocyte differentiation and its foot process formation.10 The role of Mafb in kidney disease was not established, because Mafb-deficient mice die during the perinatal period. A recent study has revealed that multicentric carpotarsal osteolysis (MCTO) is caused by mutations clustering within the amino-terminal transcriptional activation domain of MAFB. MCTO is a rare skeletal dysplasia characterized by aggressive osteolysis, particularly affecting the carpal and tarsal bones, and it is frequently associated with progressive renal failure.11 Zankl et al.11 speculated that the pathogenesis of MCTO might be attributed to MAFB dysfunction in osteoclasts and podocytes. A decade ago, Fan et al.12 identified a susceptibility locus for albuminuria by linkage analysis in diabetic KKT/a mice. Fan et al.12 identified the Mafb gene in the vicinity of the susceptible locus and observed decreased expression of its product in the kidneys.
On the basis of these facts, we hypothesized that overexpression of Mafb in podocytes would prevent the development of diabetic nephropathy. In this study, we have generated transgenic mice that overexpress Mafb in podocytes using the Nephrin promoter and enhancer. Subsequently, we induced diabetic nephropathy in these transgenic mice.
Results
Generation of Mafb Transgenic Mice That Express Mafb Specifically in Podocytes
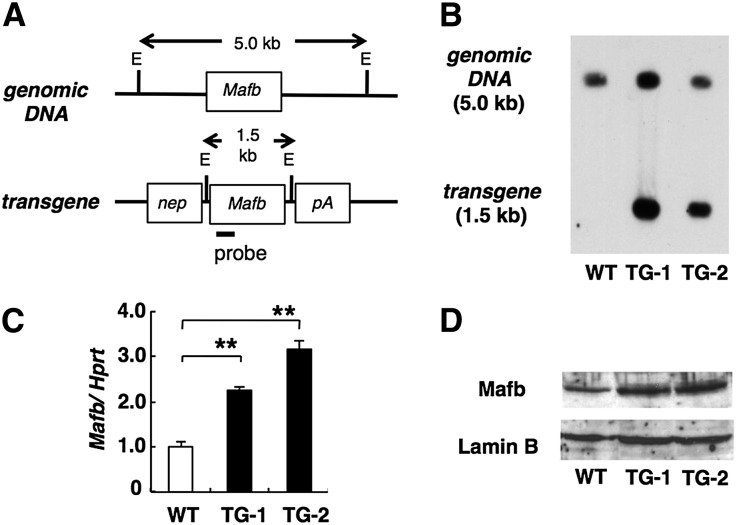
To generate transgenic (TG) mice that express high levels of Mafb specifically in podocytes, the murine Mafb coding sequence was inserted into a vector that contained the Nephrin promotor/enhancer13 (Figure 1A). We generated two TG mice lines on a C57BL/6J genetic background. Genomic DNA was analyzed by Southern blot to confirm the integrity and copy number of the transgene in each TG line. The EcoRI fragment that contained the Mafb transgene was 1.5 kb in length, whereas the corresponding fragment for the endogenous Mafb gene was 5.0 kb (Figure 1B). The results of densitometric analyses revealed that TG-1 and TG-2 each contained approximately four copies of the transgene (Figure 1B). These transgenes were stably transmitted to the progeny. To confirm that the transgenes were expressed, the levels of Mafb mRNA and protein in kidneys from TG mice were monitored by RT-PCR and Western blot (Figure 1, C and D). Mafb mRNA was overexpressed in kidneys of TG mice (Figure 1C). Consistent with this finding, Western blot analysis confirmed that the level of Mafb protein was also elevated. By the densitometry analysis, Mafb/LaminB ratio was 1.4 in TG-1, 1.6 in TG-2, and 1.0 in wild-type (WT) kidney (Figure 1D). We used TG-2 mice in the subsequent analysis.
Figure 1.
Mafb overexpression was observed in podocyte-specific Mafb transgenic kidneys. (A) Schematic of the Nephrin promoter-Mafb transgene construct. The Mafb cDNA was inserted into a vector that contained the human Nephrin promoter (Nep) and SV40 polyA (pA). The probe for Southern blot analysis, the restriction enzyme site (EcoRI [E]), and the predicted sizes of the endogenous gene and the transgene are indicated. (B) The 5.0-kb endogenous and 1.5-kb transgene fragments are shown from TG mice. (C) Analysis of Mafb mRNA in kidneys. The amount of Mafb mRNA in the samples from TG mice (TG-1 and TG-2) was higher than that in a sample from WT mice. Each bar represents the mean±SEM. **P<0.01. (D) Western blot analysis of kidneys from WT and Mafb TG mice (TG-1 and TG-2). Expression of Mafb protein was elevated in TG mice. Hprt, hypoxanthine-guanine phosphoribosyltransferase.
Podocyte-Specific Mafb Overexpression Did Not Affect Blood Glucose, Insulin Levels, or Body Weight in Streptozotocin-Induced Diabetic Mice
Hyperglycemia was induced in 8-week-old mice by intraperitoneal injection of streptozotocin (STZ). After 12 weeks of hyperglycemia, the nonfasting blood glucose levels were 509.2±26.1 mg/dl in diabetic WT (WT STZ; n=10) and 537.7±29.8 mg/dl in diabetic Mafb TG (TG STZ) mice (n=10). There was no significant difference between blood glucose concentrations in WT STZ and TG STZ mice (Table 1). We also found that insulin concentrations and body weights were not significantly different between WT STZ and TG STZ animals (Table 1). We, thus, confirmed that Mafb overexpression did not affect diabetes induction by STZ.
Table 1.
Podocyte-specific overexpression of Mafb had no effect on body weight, blood glucose, or insulin levels in either diabetic or control mice
| WT CON (n=9) | TG CON (n=9) | WT STZ (n=10) | TG STZ (n=10) | |
|---|---|---|---|---|
| Blood glucose, mg/dl (20 weeks) | 170.7±7.0 | 175.3±18.3 | 509.2±26.1a | 537.7±29.8b |
| Insulin, ng/ml (20 weeks) | 1.05±0.08 | 0.99±0.08 | 0.34±0.03a | 0.30±0.03b |
| Body weight, g (8 weeks) | 22.6±0.8 | 21.9±0.7 | 22.7±0.6 | 22.4±0.7 |
| Body weight, g (20 weeks) | 28.8±1.1 | 30.1±0.7 | 24.2±0.8a | 26.8±1.1c |
Values represent the means±SEMs.
P<0.01 versus WT CON.
P<0.01 versus TG CON.
P<0.05 versus TG CON.
Diabetic Nephropathy Was Ameliorated by Podocyte-Specific Mafb Overexpression
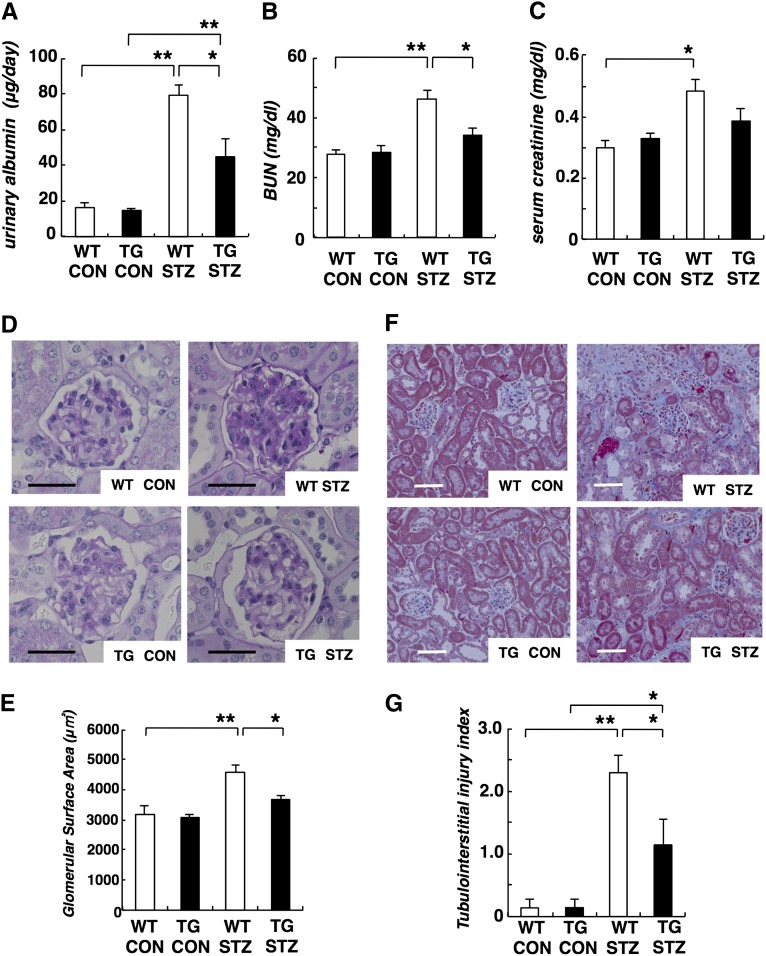
Next, we evaluated diabetic nephropathy in TG STZ mice. The level of urinary albumin in TG STZ mice was significantly lower than that of WT STZ mice at 20 weeks of age (Figure 2A). We also examined renal function in TG STZ mice by measuring BUN and serum creatinine as an index of renal function. The levels of BUN and serum creatinine were decreased in TG STZ mice compared with WT STZ mice, but the difference only reached significance in the case of BUN levels (Figure 2, B and C). Renal histopathology was also performed. In WT STZ mice, both glomerular hypertrophy and mesangial matrix expansion were observed (Figure 2D). Meanwhile, mesangial matrix expansion was less obvious in TG STZ mice (Figure 2D). Furthermore, the mean glomerular surface of TG STZ mice was significantly smaller than that of WT STZ mice (Figure 2E). We also found that renal interstitial damage was less obvious in TG STZ mice compared with WT STZ animals. (Figure 2, F and G).
Figure 2.
Diabetic nephropathy was improved in Mafb TG mice. (A) Urinary albumin, (B) BUN, and (C) serum creatinine were suppressed in TG STZ mice compared with WT STZ animals. (D) Periodic acid–Schiff-stained glomeruli. Glomerular hypertrophy and mesangial matrix expansion, which were observed in WT STZ mice, were ameliorated in TG STZ mice. Scale bar, 50 μm. (E) The mean glomerular surface area in TG STZ mice was smaller than that in WT STZ mice. (F) In the Masson’s trichrome staining, the blue area, which shows fibrosis, was smaller in TG STZ kidney compared with WT STZ kidney. Scale bar, 100 μm. (G) Tubulointerstitial injury index in TG STZ mice was ameliorated compared with WT STZ animals. Values represent the means±SEMs (n=7 mice per group). *P<0.05; **P<0.01.
Mafb Overexpression Prevented Hyperglycemia-Induced Mafb and Nephrin Depletion
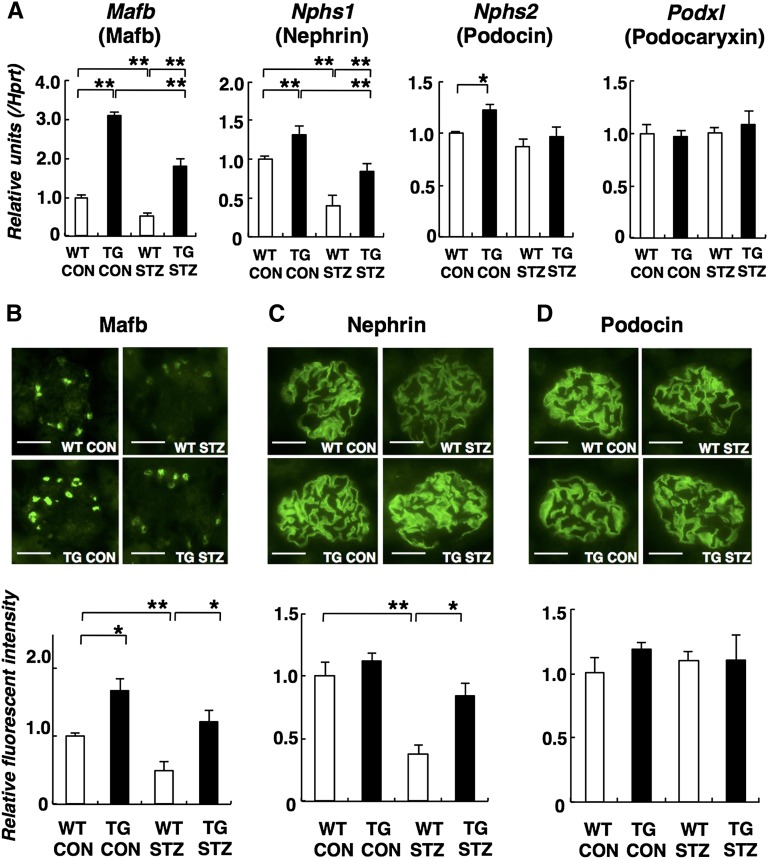
To gain insight into the molecular mechanism by which diabetic nephropathy is ameliorated in Mafb TG mice, we monitored mRNA expression from Mafb and podocyte-specific genes in glomeruli using a quantitative RT-PCR assay (Figure 3A). In nondiabetic TG (TG CON) glomeruli, increased expression of Mafb was observed. Mafb expression was reduced after induction of hyperglycemia in WT STZ glomeruli. However, Mafb expression in TG STZ glomeruli remained greater than that observed in nondiabetic WT (WT CON) mice. We also observed increased expression of Nephrin gene (Nphs1) in TG CON glomeruli. Nephrin expression is reduced during STZ-induced experimental diabetic nephropathy.14,15 Hyperglycemia-induced reduction of Nphs1 expression was also observed in WT STZ mice but partially mitigated in TG STZ glomeruli. We did not detect significant changes in the levels of expression of Nphs2 (Podocin) or Podxl (Podocaryxin) (Figure 3A).
Figure 3.
Hyperglycemia-induced Mafb and Nephrin depletion was prevented in Mafb TG mice. (A) Quantitative RT-PCR analysis. Mafb and Nphs1 elevation was observed in TG CON glomeruli. Reduced expression of Mafb and Nphs1 in WT STZ glomeruli was ameliorated in TG STZ mice. Significant changes in Nphs2 and Podxl (Podocaryxin) expression were not observed. Analysis of (B) Mafb, (C) Nephrin, and (D) Podocin expression by immunofluorescence. Scale bar, 50 μm. Graphs depictin semiquantitative analysis by immunofluorescence. Mafb overexpression was observed in TG CON glomeruli. Reduction in glomerular Mafb and Nephrin expression induced by diabetes was mitigated in TG STZ mice. Podocin expression was unchanged. Values represent the means±SEMs (n=4 mice per group). *P<0.05; **P<0.01.
Expression of the podocyte proteins Mafb, Nephrin, and Podocin was examined by semiquantitative immunofluorescence analysis (Figure 3, B–D). In WT STZ mice, Mafb expression was significantly decreased compared with the level observed in WT CON mice (Figure 3B). TG STZ mice, in contrast, exhibited no significant difference in Mafb expression compared with their TG controls. Similarly, Nephrin expression was significantly reduced in WT STZ mice compared with WT CON mice, whereas Nephrin levels did not significantly differ between TG CON and TG STZ mice (Figure 3C). We did not observe changes in Podocin expression (Figure 3D).
Mafb Directly Regulates Nephrin
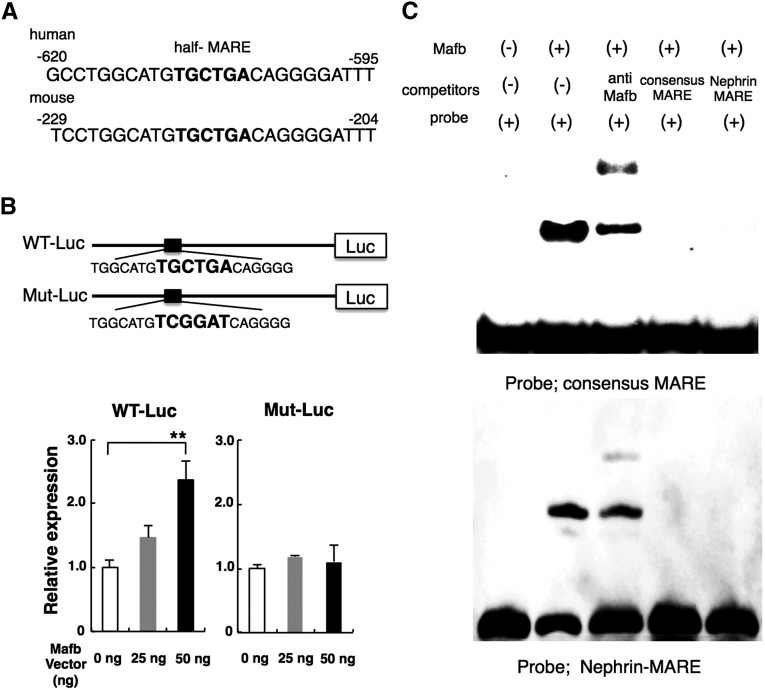
We observed a significant reduction in the expression of Nphs1 (Nephrin) mRNA in WT STZ mice, which was mitigated in TG STZ animals, suggesting a role for Mafb in the regulation of Nephrin mRNA expression. A 186-bp fragment of the human Nephrin promoter is capable of directing podocyte-specific expression of a β-galactosidase transgene when placed in front of a heterologous minimal promoter in TG mice.16 In a separate paper, Moeller et al.17 were able to show podocyte-specific expression using 1.25 kb of the proximal murine Nephrin promoter. We found that this Nephrin promoter region contains a half-MARE (TGCTGA) that is highly conserved between human and mouse (Nephrin-MARE) (Figure 4A). Therefore, we hypothesized that Mafb directly binds the Nephrin-MARE and activates Nephrin transcription. To test this hypothesis, we transfected a firefly luciferase reporter plasmid construct, which contained the murine Nephrin promoter region,18 along with the Mafb expression plasmid into 293T cells. As expected, the reporter was activated by coexpression of Mafb in a dose-dependent manner (Figure 4B). However, luciferase reporter plasmid construct that contained mutations in the Nephrin-MARE within the Nephrin promoter was not activated by Mafb (Figure 4B). Electrophoretic mobility shift assays (EMSAs) showed that nonlabeled consensus MARE and Nephrin-MARE probes competed for binding to Mafb with the biotin-labeled consensus MARE or Nephrin-MARE probes (Figure 4C). These results suggest that Mafb directly regulates the transcription of Nephrin by interacting with the highly conserved Nephrin-MARE site in its promoter region.
Figure 4.
Mafb directly regulates Nephrin expression. (A) Analysis of the 5′-flanking region of Nephrin by the Ensembl Genome Database Web site identified an Mafb binding site (half-MARE) that is highly conserved between mouse and human (Nephrin-MARE). (B) Reporter assay. WT Nephrin-MARE (WT-Luc) and mutated Nephrin-MARE (Mut-Luc) luciferase reporter constructs are indicated. The 293T cells were cotransfected with the indicated Nephrin promoter–reporter plasmid constructs along with the Mafb expression plasmid, and the relative luciferase activity was measured as described in Concise Methods. The relative luciferase activity of the reporter plasmids is shown in the lower panel with the activity generated from cells transfected with the empty reporter vector (0 ng) and the Mafb expression plasmid defined as 1.0. Each bar represents the mean±SEM. **P<0.05. (C) EMSA using labeled consensus MARE (upper panel) and Nephrin-MARE (lower panel). (Lane 1) Biotin-labeled consensus MARE probe. (Lane 2) Mafb protein and biotin-labeled consensus MARE probe. (Lane 3) Lane 2+anti-Mafb antibody. (Lane 4) Lane 2+unlabeled consensus MARE oligonucleotide. (Lane 5) Lane 2+unlabeled Nephrin-MARE oligonucleotide.
Mafb Overexpression Decreased Oxidative Stress by Gluthatione Peroxidase Elevation in Diabetic Glomeruli
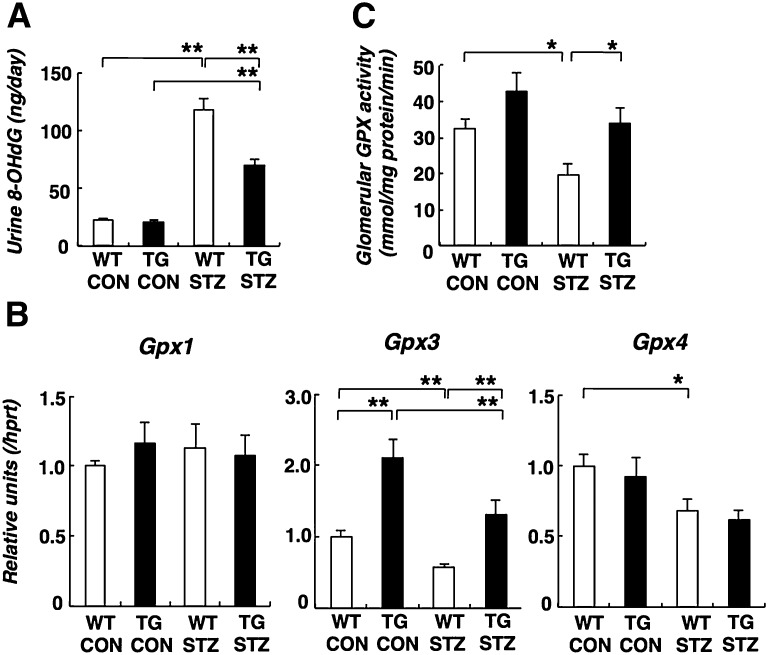
Podocytes are known to be susceptible to oxidative damage, and diabetes increases oxidative stress.1,5 We evaluated oxidative stress by measuring the urinary excretion of 8-hydroxy-2-deoxyguanosine (8-OHdG) as a marker for oxidative DNA damage in vivo. After diabetes induction, urine 8-OHdG was increased in WT STZ mice. The increased 8-OHdG excretion was prevented in TG STZ animals (Figure 5A). Maf (c-Maf), a homolog of Mafb, is a transcriptional regulator of gluthatione peroxidase 3 (Gpx3), and it modulates the antioxidative pathway in the renal proximal tubule.19 In addition, Gpx3 is also expressed in isolated glomeruli.20 Recent data suggested that Gpx3 is produced by podocytes and interacts with podocin.21 Moreover, glomerular Gpx is decreased in patients with FSGS and rats with puromycin aminonucleoside-induced nephropathy.22 These facts suggest that podocytes Gpx might play a protective role against oxidative stress in diabetic nephropathy. Of the known Gpx isoforms, Gpx1 and Gpx4 are readily found in the kidney, whereas Gpx2 and Gpx5 are not.23 RT-PCR analysis revealed decreased Gpx3 and Gpx4 expression in the WT STZ glomeruli (Figure 5B). In TG CON glomeruli, Gpx3 expression was increased approximately 2-fold. Diabetes induction reduced Gpx3 expression to a similar overall percentage, although the level in TG STZ was similar to that in WT CON glomeruli. Decreased Gpx4 expression was also seen in WT STZ glomeruli. However, there was no difference between Gpx4 expression in WT STZ and TG STZ glomeruli. We could not detect obvious changes in Gpx1 expressions. STZ causes a reduction in Gpx activity in both WT and TG mice, but the level in TG STZ mice is, nevertheless, similar to that in WT CON mice (Figure 5C).
Figure 5.
Oxidative stress was improved through Gpx activity in diabetic Mafb TG mice. (A) The urinary 8-OHdG level in TG STZ mice was significantly decreased compared with WT STZ mice. (B) Gpx expression in diabetic glomeruli by quantitative RT-PCR analysis. Gpx3 expression was increased in TG CON glomeruli. The diabetes-induced reduction in Gpx3 expression was mitigated in TG STZ glomeruli. (C) Glutathione peroxidase activity in the glomeruli. The reduction in Gpx activity in WT STZ was mitigated in TG STZ glomeruli. Values represent the means±SEMs (n=4 mice per group). *P<0.05; **P<0.01.
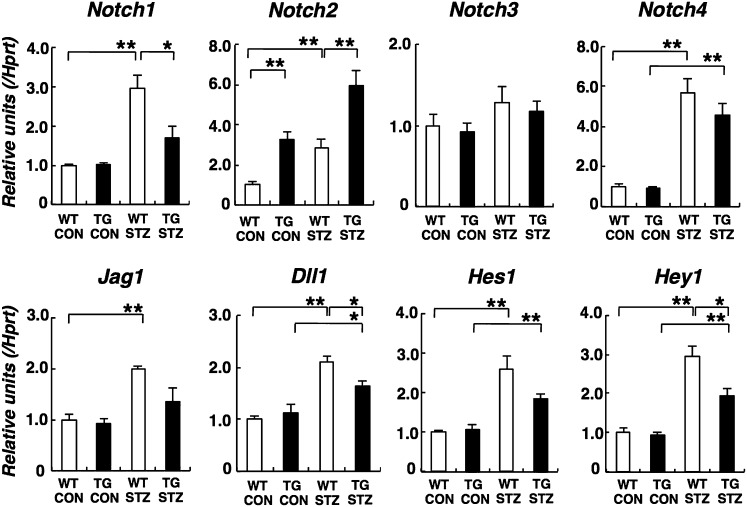
Podocyte-Specific Mafb Overexpression Altered Glomerular Notch Signaling in Diabetic Mice
Translocation and/or overexpression of MAFB have been observed in some cases of human multiple myeloma, and NOTCH2 was identified as a primary MAFB target gene in multiple myeloma cell lines.24 Recent studies described increased podocyte Notch activity in proteinuric kidney diseases and suggest its contribution to the pathogenesis of proteinuria and diabetic nephropathy.25,26 In this study, we investigated glomerular Notch pathways in diabetic Mafb TG mice (Figure 6). We found that glomerular Notch2 expression was increased in TG CON mice. However, the expression of other Notch subtypes was similar in WT and TG CON glomeruli. Increased expression of Notch1, Notch2, and Notch4 was observed in WT STZ glomeruli. Moreover, Notch1 elevation was mitigated in TG STZ glomeruli. Meanwhile, Notch2 expression was further potentiated after diabetes induction (Figure 6). There was no difference between Notch4 expression in WT STZ and TG STZ glomeruli. The expressions of Notch ligands (Jag1 and Dll1) and Notch target genes (Hes1 and Hey1) were elevated in WT STZ glomeruli. The fold induction of these genes was reduced in TG STZ glomeruli (Figure 6).
Figure 6.
Notch2 expression was increased in diabetic glomeruli, and this effect was enhanced in diabetic Mafb TG glomeruli. Analysis of Notch signaling expression by quantitative RT-PCR in diabetic Mafb TG glomeruli. We observed increased expression of Notch1 and Notch4, Notch ligands (Jag1 and Dll1), and Notch target genes (Hes1 and Hey1) in WT STZ glomeruli. The fold induction of these genes was mitigated in TG STZ glomeruli. Meanwhile, glomerular Notch2 expression was increased in TG CON mice. Notch2 expression was further potentiated in TG STZ mice. Values represent the means±SEMs (n=4 mice per group). *P<0.05; **P<0.01.
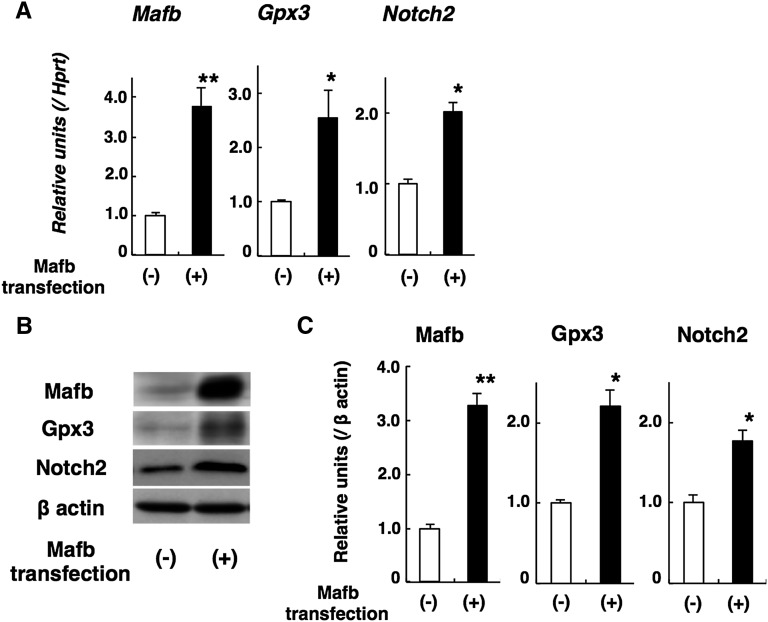
Mafb Induces Gpx3 and Notch2 Expression in Cultured Podocytes
We observed upregulation of Gpx3 and Notch2 in Mafb TG glomeruli, suggesting a role for Mafb in the regulation of Gpx3 and Notch2 expression. It was reported that c-Maf induces Gpx3 in renal tubule epithelial cells,19 and c-Maf or Mafb induces Notch2 expression in myeloma cells.24 However, there is no proof that Mafb regulates Gpx3 and Notch2 expression in podocytes. Therefore, we transfected murine podocytes with an Mafb expression vector or performed a mock transfection as a control. We observed elevated Gpx3 and Notch2 expression in Mafb-transfected podocytes by RT-PCR (Figure 7A) and Western blot analyses (Figure 7, B and C).
Figure 7.
Mafb upregulates Gpx3 and Notch2 expression in cultured podocytes. (A) Quantitative RT-PCR analysis. Gpx3 and Notch2 elevation was observed in Mafb-transfected podocytes. (B) Representative Western blotting of Mafb, Gpx3, and Notch2. (C) Graph depicting quantitative analysis by Western blot. Gpx3 and Notch2 elevation was found in Mafb-transfected podocytes. Values represent the means±SEMs (n=3 per group in independent experiments). *P<0.05; **P<0.01.
We also observed downregulation of Mafb and Gpx3 and upregulation of Notch2 in diabetic glomeruli. Additionally, we cultured Mafb-transfected and nontransfected podocytes in high glucose conditions (30 mM glucose). However, we did not observe any apparent change in the relative levels of Mafb, Gpx3, or Notch2 expression in cells cultured in media containing normal glucose (5.5 mM) versus high glucose (30 mM) for 72 hours (data not shown). Because hyperglycemia in STZ-treated diabetic mice persisted for 12 weeks, the difference between the in vivo and in vitro analyses might have arisen owing to the high glucose duration.
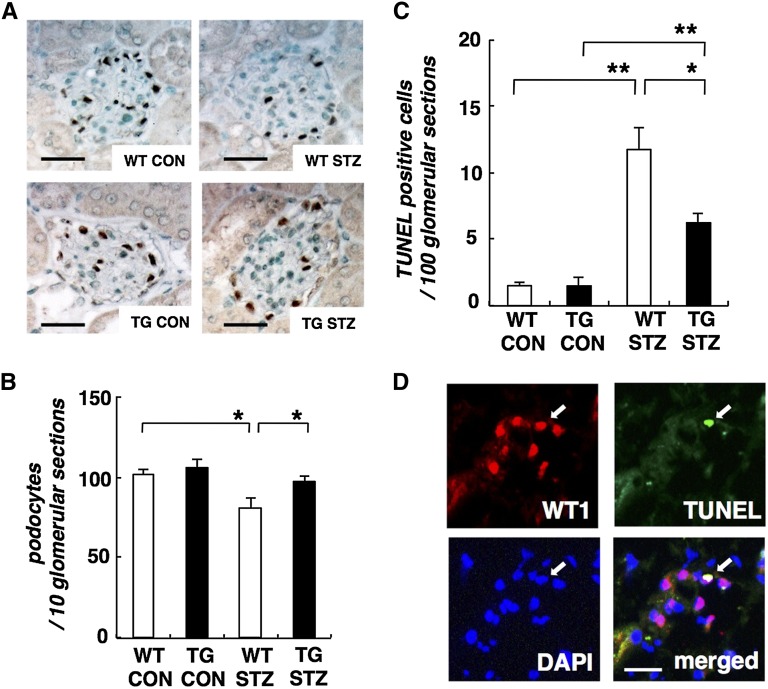
Overexpression of Mafb Restores Podocyte Depletion and Apoptosis Induced by Diabetes
Loss of podocytes contributes importantly to the pathogenesis of diabetic nephropathy.1 We assayed the podocyte numbers by counting glomerular nuclei staining positively for Wilms’ tumor 1 (WT1). The number of podocytes per glomerular section was reduced in WT STZ mice compared with WT CON animals. In contrast, podocyte depletion was mitigated in TG STZ mice (Figure 8, A and B).
Figure 8.
Overexpression of Mafb partially restores podocyte number. (A) Typical images of WT1-stained glomeruli from WT CON, TG CON, WT STZ, and TG STZ mice. (B) Average number of WT1-stained nuclei calculated per glomerular sections. The number of WT1-stained nuclei was obtained from 10 glomerular sections per kidney sample. Values represent the means±SEMs (n=4 mice per group). *P<0.05. (C) Diabetes-induced glomerular cell death is reduced by Mafb overexpression. TUNEL staining in WT CON, TG CON, WT STZ, and TG STZ glomeruli. Quantification of TUNEL staining in all glomerular sections per kidney sample. Values represent the means±SEMs (n=4 mice per group). *P<0.05; **P<0.01. (D) Apoptotic glomerular cells were podocytes in diabetic glomeruli. TUNEL-positive apoptotic podocyte in the glomeruli of WT STZ mice was WT1-positive (arrows). Blue, 4′,6-diamidino-2-phenylindole (DAPI); green, TUNEL; red, WT1. Scale bar, 50 μm.
Podocyte apoptosis has been identified as one cause of podocyte depletion in experimental kidney diseases in mice.3–5 To determine whether the reduction in the apparent number of podocytes was related to increased cell death, sections were subjected to terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining. Apoptotic glomerular cells were rare in glomerular sections from control mice. TUNEL-positive glomerular cells were increased in WT STZ mice, and this induction of cell death was significantly decreased in TG STZ mice (Figure 8C). We also confirmed that apoptotic glomerular cells were WT1-positive, indicating that they were podocytes (Figure 8D).
Discussion
We previously showed that Mafb is essential for differentiation and foot process formation of podocytes.10 A recent study reported that MAFB mutations cause MCTO. Patients with MCTO often progress to end stage kidney disease.11 Because some of the cases showed FSGS,27 renal failure might be attributed to podocyte dysfunction caused by MAFB mutations.11 The Mafb gene has been reported to localize to the vicinity of the albuminuria-susceptible locus in diabetic mice.12 These facts suggest Mafb might play an important role in diabetic nephropathy. In this study, we analyzed diabetic Mafb TG mice. We found that Mafb overexpression might ameliorate diabetic nephropathy through Nephrin, Gpx3, and Notch2 in podocytes.
Nephrin, the key molecule of the glomerular slit diaphragm, is expressed on the surface of podocytes and critical in preventing proteinuria.1 In diabetes, hyperglycemia leads to the loss of surface expression of Nephrin and causes proteinuria in human diabetic nephropathy and rodent STZ-induced diabetic model.28,29 Although the mechanism of Nephrin loss remains unclear, hyperglycemia induces protein kinase C α-mediated Nephrin endocytosis.30 In this study, we found that Mafb overexpression significantly attenuated Nephrin loss in diabetic mice. Moreover, it was observed that Mafb stimulates Nephrin transcription through an MARE within the proximal promoter of the Nephrin gene. Several studies have shown that Nephrin is regulated at the transcriptional level.14,16,17 Guo et al.16 reported that a 186-bp fragment of the human Nephrin promoter containing retinoid X receptor/retinoic acid receptor and WT1 response elements is capable of directing podocyte-specific expression. An MARE is also located within this fragment. Our data suggest that Mafb signaling is an important regulator of the Nephrin promoter. However, we could not identify MAREs within the 5′-flanking regions of Gpx3 or Notch2. The mechanism by which Mafb regulates Gpx3 and Notch2 expressions thus remains to be determined. Additional studies and other supporting data are needed to clarify the precise biologic properties of Mafb.
Gpx, which is an antioxidative stress enzyme, was elevated in Mafb TG glomeruli. It has been suggested that oxidative stress constitutes a key and common event in the pathogenesis of diabetic nephropathy.31 Antioxidant administration has been shown to have potential beneficial effects in human and experimental diabetic nephropathy.32–35 A recent study suggested that Gpx3 is produced by podocytes and interacts with podocin.21 The mRNA expression of Gpx3 was significantly reduced in glomeruli after puromycin aminonucleoside nephropathy induction.20 Our data suggest Mafb overexpression in podocytes might ameliorate diabetic nephropathy through Gpx3.
We also found that Notch2 expression was increased in diabetic Mafb TG glomeruli. In contrast, the expression of Notch1, Notch ligands, and Notch target genes was decreased. NOTCH2 has been identified as a primary MAFB target gene.24 Several recent studies described the expression of Notch pathway proteins in kidneys of patients with diabetic nephropathy.25,36 Expression of Notch1 in podocytes causes the development of albuminuria and glomerulosclerosis, whereas genetic deletion or pharmacological inhibition ameliorates diabetic nephropathy in murine models.25 In vitro and in vivo studies showed that Notch1 induces apoptosis of podocytes through the activation of p53.25,37 Meanwhile, Notch2 elevation in podocytes has also been observed in human and experimental diabetic nephropathy.25,38 However, the in vivo role of Notch2 in podocytes is poorly understood. Increased renal tubulointerstitial Notch1 levels are associated with increased fibrosis and decreased renal function, whereas tubulointerstitial expression of Notch2 is associated with samples with less fibrosis and better renal function in CKD.38 These results would suggest that, similar to tubulointerstitial fibrosis, Notch1 and Notch2 in podocytes might have different roles in diabetic nephropathy as well. An opposing role between Notch1 and Notch2 has been observed in other diseases. For example, in colorectal cancer, high expression of Notch1 is associated with poor overall survival, whereas high expression of Notch2 predicts better survival.39 Additional studies are needed to elucidate the role of Notch2 in podocytes and the interaction between Notch1 and Notch2. A recent study showed Mafb–green fluorescent protein bacterial artificial chromosomes in TG mice. The work by Brunskill et al.40 represents microarrays of podocytes from embryos at embryonic day (E) 13.5 and E15.5 as well as adults. The report will be useful to analyze additional targets for Mafb.
Loss of podocytes contributes to the progression of diabetic nephropathy. Podocyte apoptosis is known as one cause of podocyte depletion.1 We observed that apoptotic podocytes were decreased in diabetic Mafb TG glomeruli. Notch signaling changes and antioxidant protection by Mafb might induce the antiapoptotic effects. Mafb expression has been shown to confer antiapoptotic effects in myeloma and macrophage cell lines.24,41
In summary, we showed in the present study that Mafb might play a protective role in diabetic nephropathy. This effect is regulated through slit-diaphragm proteins, antioxidative enzymes, and Notch pathways in podocytes. We, therefore, suggest that an increased Mafb activity is of pivotal importance for the development of diabetes nephropathy. Mafb could be a therapeutic target in diabetic nephropathy.
Concise Methods
Mice
We generated TG mice that overexpress Mafb in podocytes using the human Nephrin promoter/enhancer.13 Eight-week-old WT and Mafb TG mice were treated with either STZ or saline solution. After 12 weeks of treatment, diabetic nephropathy was assessed by biochemical analyses of urine, serum, and histologic analyses of the kidneys. Diabetes was induced by a single intraperitoneal injection of STZ (150 mg/kg body wt; Sigma-Aldrich, St. Louis, MO) that had been diluted in citrate buffer (pH 4.5). Control mice were injected with an equal volume of citrate buffer alone. After 7 days, the induction of diabetes was confirmed by the measurement of blood glucose concentrations. Fed animals with blood glucose levels that were greater than 300 mg/dl were considered to be diabetic. Mice were maintained in the Laboratory Animal Resource Center. All experiments were performed according to the Guide for the Care and Use of Laboratory Animals of the University of Tsukuba.
Southern Hybridization Analysis of Genomic DNA
High-molecular weight DNA was prepared from the tail of each mouse, and 25 μg DNA was digested with EcoRI and then electrophoresed through 1.0% agarose gels. After electrophoresis, the DNA was transferred to a nylon membrane (Hybond-N+; GE Healthcare Life Sciences, Little Chalfont, UK). Southern hybridization was performed using digoxigenin-labeled DNA probes that were amplified using forward (5′-TGAGCATGGGGCAAGAGCTG-3′) and reverse (5′-CCATCCAGTACAGGTCCTCG-3′) oligonucleotide primers in hybridization buffer at 42°C overnight. After hybridization, probes were detected using alkaline phosphatase-conjugated antidigoxigenin antibody (Roche Diagnostics, Basel, Switzerland). Transgene copy number was determined from the blot with ImageJ (National Institutes of Health, Bethesda, MD).
Western Blot Analysis of Kidneys
Nuclear extracts were prepared from the kidneys of 10-week-old WT or TG mice. The extracts were fractionated by size on a 10% SDS–polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (EMD Millipore Corporation, Billerica, MA), and incubated with primary and secondary antibodies. To detect the Mafb protein, a rabbit antibody against mouse Mafb (BL658; Bethyl, Montgomery, TX) was used as the primary antibody, and peroxidase-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) was used as the secondary antibody. To normalize the results with respect to the amount of protein in each sample, a goat antibody against mouse Lamin B (C-20; Santa Cruz Biotechnology, Dallas, TX) was used. Amount of proteins was determined from the blot with ImageJ.
Measurements of Serum Glucose and Insulin
The serum concentration of glucose was measured using a Dry-Chem 3500 automated analyzer for routine laboratory tests (FujiFilm Inc., Tokyo, Japan). The serum insulin levels in fed animals were measured with a rat insulin ELISA kit (Morinaga Institute of Biologic Science, Yokohama, Japan).
Analysis of Urinary Albumin Excretion and Renal Serological Assays
The urine of each mouse was collected in an individual metabolic cage over a 24-hour period. Urinary albumin was measured using the Albuwell M ELISA quantitation kit according to the protocols by the manufacturer (Exocell, Philadelphia, PA). The serum concentration of BUN and creatinine were measured using a Dry-Chem 3500 automated analyzer.
Histopathological Analysis of Renal Tissues
Each mouse was bled while under ether anesthesia. At autopsy, organs were fixed with 10% formalin in 0.01 mol/L phosphate buffer (pH 7.2) and embedded in paraffin. Sections were stained with Periodic acid–Schiff stain and Masson’s trichrome staining for histopathological examination under light microscopy. For immunofluorescence analysis, frozen sections were stained with anti-Nephrin, anti-Podocin and anti-Mafb as previously described.10 Quantitative estimation of immunofluorescence was performed using ImageJ. Relative fluorescence intensity was calculated using the mean fluorescence intensity of nondiabetic WT mice defined as 1.0.
Glomerular Surface Area Determination
The glomerular surface area of 20 glomeruli in each kidney section was measured using a BIOREVO BZ-9000 microscope (Keyence, Osaka, Japan), and the mean glomerular surface area was calculated.
Tubulointerstitial Morphology
The index of renal tubulointerstitial damage was graded with the experimental protocol using a light microscope on a scale of grades 0–4 (grade 0, normal; grade 1, affected area<10%; grade 2, affected area=10%–25%; grade 3, affected area=25%–75%; grade 4, affected area>75%) as previously described.42
Glomerular Isolation
Mice were anesthetized and perfused through the heart with magnetic 4.5-μm-diameter Dynabeads (Invitrogen). Kidneys were minced into small pieces, digested by collagenase A (Roche), filtered, and collected using a magnet.43
Quantitative RT-PCR
Total RNA (1 μg) was reverse-transcribed into cDNA. Each reaction was done in duplicate. The quantity of cDNA in each sample was normalized to the amount of Hprt cDNA. For the PCR, we used SYBR Premix Ex Taq II (TAKARA Bio) according to the manufacturer's instructions. The amplification was carried out in a Thermal Cycler Dice Real Time System (TAKARA Bio). The following primer pairs were used: Mafb, 5′-TGAGCATGGGGCAAGAGCTG-3′ and 5′-CCATCCAGTACAGGTCCTCG-3′; Nphs1, 5′-GCCACCACCTTCACACTGAC-3′ and 5′-AGACCACCAACCGCAAAGA-3′; Nphs2, 5′-GGGCATCAAAGTGGAGAGAACTG-3′ and 5′-TGGACAGCGACTGAAGAGTGTG-3′; Podxl, 5′-GAGCACAGCGAGCCATCC-3′ and 5′-GTGGAGACGGGCAATGTAG-3′; Gpx1, 5′-GGTTCGAGCCCAATTTTACA-3′ and 5′-CCCACCAGGAACTTCTCAAA-3′; Gpx3, 5′-GATGTGAACGGGGAGAAAGA-3′ and 5′-CCCACCAGGAACTTCTCAAA-3′; Gpx4, 5′-CTCCATGCACGAATTCTCAG-3′ and 5′-ACGTCAGTTTTGCCTCATTG-3′; Notch1, 5′-ATGGCATCGCGGGCTTCACTT-3′ and 5′-TCCTTGCATACCCCGCTGTTTTTG-3′; Notch2, 5′-CATTGACGAGTGCACTGA-3′ and 5′-GAGTGCTGGCACAAGTGT-3′; Notch3, 5′-GTGGATGTCAACGTCCGA-3′ and 5′-TGCCAGGATCAGTGCAGT-3′; Notch4, 5′-CAGAACGTGGATCCCCTCAAGTTGC-3′ and 5′-AGGCAGAGAGAGGGCAAGGACTCAT-3′; Jag1, 5′-CGGTGGCTGGGAAGGAA-3′ and 5′-CTCGGGCCACACCAGAC-3′; Dll1, 5′-GGCTCTTCCCCTTGTTCTAAC-3′ and 5′-CTTCAGCCGGACGCAGACC-3′; Hes1, 5′-AAAGACGGCCTCTGAGCACA-3′ and 5′-TCATGGCGTTGATCTGGGTCA-3′; Hey1, 5′-GGGAGGGTCAGCAAAGCA-3′ and 5′-GCTGCGCATCTGATTTGTCA-3′; Hprt, 5′-TTGTTGTTGGATATGCCCTTGACTA-3′ and 5′-AGGCAGATGGCCACAGGACTA -3′.44–46
Luciferase Assay
To construct reporter plasmids, DNA fragments corresponding to positions −427 to +173 in the WT and mutant murine Nephrin gene promoter18 were PCR-amplified and subcloned into the pGL3-Luc vector (Promega, Madison, WI). To express Mafb, we used a previously described expression plasmid (pEFX3-FLAG-Mafb).47 Reporter and effecter plasmids were transfected into 293T cells by lipofection using FuGENE6 transfection regent (Roche) and then harvested 24 hours post-transfection. Luciferase assays were performed according to the manufacturer's protocol using the Dual-Luciferase Reporter Assay System (Promega). Transfection efficiency was routinely monitored and normalized using the coexpressed Renilla reniformis luciferase activity, which was expressed from the pRL-TK (Promega) expression plasmid.
EMSA
Biotin labeling of oligonucleotide probes and protein–DNA binding reactions were performed using the LightShift chemiluminescent EMSA kit (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s directions. Mafb and mock-control proteins were prepared from 293T cells transiently transfected with expression vectors, pEFX3-FLAG-Mafb or pEFX3-FLAG mock, as previously described.48 Nuclear extracts were incubated with the biotin-labeled probe and a reaction mixture containing 10 mM Tris, 50 mM KCl, 1 mM dithiothreitol, 5 mM MgCl2, 50 ng/L poly (dI–dC), 0.05% NP-40, and 2.5% glycerol for 20 minutes at room temperature. The oligonucleotide probes containing the MARE sequences are as follows: consensus MARE sequence: 5′-AGCTCGGAATTGCTGACTCATCATTACTC-3′ and 3′-TCGAGCCTTAACGACTGAGTAGTAATGAG-5′; Nephrin-MARE: 5′-GTTCCTGGCATGTGCTGACAGGGGATTTC-3′ and 3′-CAAGGACCGTACACGACTGTCCCCTAAAG-5′. A 200-fold excess of unlabeled oligonucleotide was added to the reaction mixture for competition studies. Anti- Mafb antibody (BL658; Bethyl) was preincubated with the extract protein for 10 minutes before incubation of the biotin-labeled probe to detect each protein.
Measurement of Urinary Levels of 8-OHdG
The urinary excretion of 8-OHdG was measured by competitive ELISA using a commercially available kit: New 8-OHdG Check (Japan Institute for the Control of Aging, Shizuoka, Japan).
Measurement of Glutathione Peroxidase Activity
Collected glomeruli were subjected to glutathione peroxidase assays according to the manufacturer’s instructions. Glutathione peroxidase assays kits and reagents were all purchased from Cayman Chemical Company (Ann Arbor, MI).
Cell Culture
Mouse podocytes (SVI) from a conditionally immortalized cell line were obtained from CLS.49 Cells were grown in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% FBS, penicillin (100 units/L), and streptomycin (100 mg/L) in a controlled humidified atmosphere. To propagate podocytes, the culture medium was supplemented with 10 units/ml mouse recombinant IFN-γ, and the cells were cultivated at 33°C to enhance the expression of the temperature-sensitive large T antigen (permissive conditions). To induce differentiation, podocytes were maintained at 37°C without IFN-γ (nonpermissive conditions) for 7 days.50
Cell Transfection
One day before transfection, the culture medium was removed, and cells were cultivated in antibiotic-free RPMI 1640 (Gibco) supplemented with 10% FBS. The cells were transfected with expression vectors, pEFX3-FLAG-Mafb or pEFX3-FLAG mock, using Lipofectamine LTX (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. The cells were incubated an additional 24 hours. Transfected cells were cultured in normal (5.5 mM) and high (30 mM) glucose concentration for 72 hours. Subsequently, Western blot analysis and quantitative RT-PCR were performed.
Western Blot Analysis of Podocytes
Podocytes were treated with lysis buffer (20 mM Tris, 140 mM NaCl, 2 mM EDTA, 10% glycerol, and 1% Nonidet P-40) in the presence of a protease inhibitor cocktail (Sigma-Aldrich) and then homogenized at 4°C by scraping. The homogenates were centrifuged at 9500×g for 20 minutes at 4°C, and supernatants were used as sample proteins. Equal amounts of protein, containing 20 μg, were separated on a SDS–polyacrylamide gel (10%) and transferred to Immobilon-P PVDF Membrane (EMD Millipore). The following antibodies were used for Western blotting: anti-Mafb (Bethyl), anti-Gpx3 (H-10; Santa Cruz Biotechnology), anti-Notch2 (D76A6, Cell Signal Technology, Danvers, MA), and anti–β-actin (horseradish peroxidase–conjugate; 13E5; Cell Signaling Technology). Blots were developed by using the Pierce Fast Western Blot Kit, and the signals were captured on an image reader (Las-3000; FujiFilm, Tokyo, Japan). Amount of proteins was determined from the blot with ImageJ.
Podocyte Number Counting
Kidney sections (4 μm) were immunostained with a polyclonal antibody against WT1 (C-19; Santa Cruz Biotechnology) as the primary antibody and detected by the avidin-biotin-peroxidase complex staining technique using a Histofine Kit (Nichirei, Tokyo, Japan). Podocyte density was determined from a count of the number of WT1-positive nuclei per glomerular section.
TUNEL Assay
Apoptotic cells were estimated by the TUNEL assay, which relies on incorporation of labeled 2′-deoxyuridine, 5′-triphosphate at sites of DNA breaks. For the TUNEL procedure, all reagents, including buffer, were part of a kit (In Situ TAKARA Apoptosis Kit; TAKARA Bio) applied to frozen kidney sections. Procedures were carried out according to the manufacturer’s instructions. For identification of apoptotic podocytes, the kidney sections were costained with WT1 antibody (C-19; Santa Cruz Biotechnology) and 4′,6-diamidino-2-phenylindole. Apoptosis was examined in 4-μm-thick sections of the kidneys. For quantitative histologic analysis, the degree of apoptosis was estimated using a scale based on the mean number of TUNEL-positive cells per 100 glomerular sections.
Statistical Analyses
All results were expressed as means±SEMs. Multiple data comparisons were conducted using the one-way ANOVA with the Bonferroni correction. Significant differences between the two groups of mice were analyzed using the unpaired t test. Differences were considered statistically significant at P values <0.05.
Disclosures
None.
Acknowledgments
This work was supported by The Japan Society for the Promotion of Science KAKENHI Grant-in-Aid for Scientific Research 24591223.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Jefferson JA, Shankland SJ, Pichler RH: Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int 74: 22–36, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP: Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Susztak K, Raff AC, Schiffer M, Böttinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 6.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Fredersdorf S, Weil J, Ulucan C, Birner C, Büttner R, Schubert T, Böger CA, Debl K, Muders F, Riegger GA, Luchner A: Vasopeptidase inhibition attenuates proteinuria and podocyte injury in Zucker diabetic fatty rats. Naunyn Schmiedebergs Arch Pharmacol 375: 95–103, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kataoka K, Fujiwara KT, Noda M, Nishizawa M: MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol Cell Biol 14: 7581–7591, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka K, Noda M, Nishizawa M: Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol 14: 700–712, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S: MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol 26: 5715–5727, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zankl A, Duncan EL, Leo PJ, Clark GR, Glazov EA, Addor MC, Herlin T, Kim CA, Leheup BP, McGill J, McTaggart S, Mittas S, Mitchell AL, Mortier GR, Robertson SP, Schroeder M, Terhal P, Brown MA: Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB. Am J Hum Genet 90: 494–501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Q, Shike T, Shigihara T, Tanimoto M, Gohda T, Makita Y, Wang LN, Horikoshi S, Tomino Y: Gene expression profile in diabetic KK/Ta mice. Kidney Int 64: 1978–1985, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Wong MA, Cui S, Quaggin SE: Identification and characterization of a glomerular-specific promoter from the human nephrin gene. Am J Physiol Renal Physiol 279: F1027–F1032, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Menne J, Meier M, Park JK, Boehne M, Kirsch T, Lindschau C, Ociepka R, Leitges M, Rinta-Valkama J, Holthofer H, Haller H: Nephrin loss in experimental diabetic nephropathy is prevented by deletion of protein kinase C alpha signaling in-vivo. Kidney Int 70: 1456–1462, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD: Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo G, Morrison DJ, Licht JD, Quaggin SE: WT1 activates a glomerular-specific enhancer identified from the human nephrin gene. J Am Soc Nephrol 15: 2851–2856, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Deb DK, Wang Y, Zhang Z, Nie H, Huang X, Yuan Z, Chen Y, Zhao Q, Li YC: Molecular mechanism underlying 1,25-dihydroxyvitamin D regulation of nephrin gene expression. J Biol Chem 286: 32011–32017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirota S, Yoshida T, Sakai M, Kim JI, Sugiura H, Oishi T, Nitta K, Tsuchiya K: Correlation between the expression level of c-maf and glutathione peroxidase-3 in c-maf -/- mice kidney and c-maf overexpressed renal tubular cells. Biochem Biophys Res Commun 348: 501–506, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Granqvist A, Nilsson UA, Ebefors K, Haraldsson B, Nyström J: Impaired glomerular and tubular antioxidative defense mechanisms in nephrotic syndrome. Am J Physiol Renal Physiol 299: F898–F904, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Lee BH, Kim DJ: Identification of proteins that interact with podocin using the yeast 2-hybrid system. Yonsei Med J 50: 273–279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HC, Guh JY, Lai YH: Alterations of glomerular and extracellular glutathione peroxidase levels in patients and rats with focal segmental glomerulosclerosis. J Lab Clin Med 137: 279–283, 2001 [DOI] [PubMed] [Google Scholar]
- 23.de Haan JB, Stefanovic N, Nikolic-Paterson D, Scurr LL, Croft KD, Mori TA, Hertzog P, Kola I, Atkins RC, Tesch GH: Kidney expression of glutathione peroxidase-1 is not protective against streptozotocin-induced diabetic nephropathy. Am J Physiol Renal Physiol 289: F544–F551, 2005 [DOI] [PubMed] [Google Scholar]
- 24.van Stralen E, van de Wetering M, Agnelli L, Neri A, Clevers HC, Bast BJ: Identification of primary MAFB target genes in multiple myeloma. Exp Hematol 37: 78–86, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K: The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 14: 290–298, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Sirin Y, Susztak K: The story of Notch and chronic kidney disease. Curr Opin Nephrol Hypertens 20: 56–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor A, Highton J, Hung NA, Dunbar J, MacGinley R, Walker R: Multicentric carpal-tarsal osteolysis with nephropathy treated successfully with cyclosporine A: A case report and literature review. Am J Kidney Dis 50: 649–654, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G: Nephrin expression is reduced in human diabetic nephropathy: Evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 52: 1023–1030, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Bonnet F, Tikellis C, Kawachi H, Burns WC, Wookey PJ, Cao Z, Cooper ME: Nephrin expression in the post-natal developing kidney in normotensive and hypertensive rats. Clin Exp Hypertens 24: 371–381, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Quack I, Woznowski M, Potthoff SA, Palmer R, Königshausen E, Sivritas S, Schiffer M, Stegbauer J, Vonend O, Rump LC, Sellin L: PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem 286: 12959–12970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Obrosova IG, Fathallah L, Liu E, Nourooz-Zadeh J: Early oxidative stress in the diabetic kidney: Effect of DL-alpha-lipoic acid. Free Radic Biol Med 34: 186–195, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Gui D, Guo Y, Wang F, Liu W, Chen J, Chen Y, Huang J, Wang N: Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS ONE 7: e39824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderón-Salinas JV, Muñoz-Reyes EG, Guerrero-Romero JF, Rodríguez-Morán M, Bracho-Riquelme RL, Carrera-Gracia MA, Quintanar-Escorza MA: Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol Cell Biochem 357: 171–179, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Miao J, Wang S, Zhang Y: The protective effects of beta-casomorphin-7 against glucose -induced renal oxidative stress in vivo and vitro. PLoS ONE 8: e63472, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn SH, Susztak K: Getting a notch closer to understanding diabetic kidney disease. Diabetes 59: 1865–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waters AM, Wu MY, Huang YW, Liu GY, Holmyard D, Onay T, Jones N, Egan SE, Robinson LA, Piscione TD: Notch promotes dynamin-dependent endocytosis of nephrin. J Am Soc Nephrol 23: 27–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K: Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int 78: 514–522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu D, Zhang Z, Zhou Y, Wang W, Li Y, Zhang H, Dong G, Zhao Q, Ji G: Notch1 and Notch2 have opposite prognostic effects on patients with colorectal cancer. Ann Oncol 22: 2440–2447, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS: Defining the molecular character of the developing and adult kidney podocyte. PLoS ONE 6: e24640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machiya J, Shibata Y, Yamauchi K, Hirama N, Wada T, Inoue S, Abe S, Takabatake N, Sata M, Kubota I: Enhanced expression of MafB inhibits macrophage apoptosis induced by cigarette smoke exposure. Am J Respir Cell Mol Biol 36: 418–426, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Ren XJ, Guan GJ, Liu G, Zhang T, Liu GH: Effect of activin A on tubulointerstitial fibrosis in diabetic nephropathy. Nephrology (Carlton) 14: 311–320, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes KM, Evenson JK, Raines AM, Sunde RA: Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr 139: 199–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sunde RA, Raines AM, Barnes KM, Evenson JK: Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep 29: 329–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyfeler Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U, Taylor V: Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J 24: 3504–3515, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kajihara M, Sone H, Amemiya M, Katoh Y, Isogai M, Shimano H, Yamada N, Takahashi S: Mouse MafA, homologue of zebrafish somite Maf 1, contributes to the specific transcriptional activity through the insulin promoter. Biochem Biophys Res Commun 312: 831–842, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Nakamura M, Hamada M, Hasegawa K, Kusakabe M, Suzuki H, Greaves DR, Moriguchi T, Kudo T, Takahashi S: c-Maf is essential for the F4/80 expression in macrophages in vivo. Gene 445: 66–72, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Schiwek D, Endlich N, Holzman L, Holthöfer H, Kriz W, Endlich K: Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Piwkowska A, Rogacka D, Audzeyenka I, Jankowski M, Angielski S: High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J Cell Biochem 112: 1661–1672, 2011 [DOI] [PubMed] [Google Scholar]