Abstract
In addition to their role in the digestion and absorption of dietary fats, bile acids (BAs) are tightly regulated signalling molecules. Their levels in the intestinal lumen, circulation and tissues fluctuate after feeding and fasting, and as a result of certain diseases and therapies. BAs regulate many cell types in the gut wall and beyond by activating nuclear and plasma membrane receptors. Of these, the G protein-coupled receptor TGR5 has emerged as a key mediator of the non-genomic actions of BAs. TGR5 is a cell-surface receptor that couples to Gαs, formation of cAMP, activation of protein kinase A and extracellular signal-regulated kinases, and inhibition of inflammatory signalling pathways. TGR5 has been implicated in mediating the actions of BAs on secretion of glucagon-like peptide 1 and glucose homeostasis, gastrointestinal motility and transit, electrolyte and fluid transport in the colon, bile formation and secretion, sensory transduction and inflammation. TGR5 agonists have been developed as treatments for metabolic, inflammatory and digestive disorders, and emerging evidence suggests that TGR5 mutations are associated with inflammatory diseases. Thus, TGR5 plays an important role in the normal processes of digestion and is a new therapeutic target for important digestive diseases.
 |
Nigel Bunnett is a National Health and Medical Research Council Australia Fellow, Professor of Pharmacology and Medicine, and Deputy Director of the Monash Institute of Pharmaceutical Sciences. He obtained a PhD from the University of Cambridge, spent 25 years at the University of California, San Francisco, and joined Monash University in 2011. Nigel's research investigates the signalling mechanisms that underlie pain, itch and neurogenic inflammation. He is recognized for his work on defining the functions and regulation of G protein-coupled receptors and transient receptor potential ion channels, major cell-surface proteins that are essential for sensory transduction and neurogenic inflammation.
Introduction
Bile acids (BAs), the main component of bile, are a large and complex family of amphipathic molecules synthesized from cholesterol. They show remarkable structural heterogeneity between species and within the same species in different tissues, compartments and patho-physiological states (reviewed in Hofmann & Hagey, 2008). Their structural diversity is matched by functional diversity, including the digestion and absorption of dietary fats and hormone-like effects through the activation of nuclear and plasma membrane receptors. This review concerns the neuro-humoral actions of BAs within the gastrointestinal system that are mediated by the plasma membrane receptor TGR5 (GpBAR1, M-BAR, GPBA, GPR131). TGR5 mediates the actions of BAs on disparate patho-physiological processes, ranging from glucose homeostasis to peristalsis, and TGR5 is a therapeutic target for disorders of metabolism, inflammation, digestion and sensation (reviewed in Thomas 2008; Lieu et al. 2014).
BAs are regulated messenger molecules
The concentrations of bile acids within the intestinal lumen and general circulation fluctuate during physiological states, and can be affected by disease and therapy (Fig. 1). In humans, the principal primary BAs, cholic acid (CA) and chenodeoxycholic acid (CDCA), are synthesized from cholesterol in hepatocytes. Prior to secretion into the canalicular lumen, they are conjugated to glycine or taurine, which increases their hydrophobicity. Bile is secreted into the intestine during feeding. By virtue of their amphipathic nature, BAs aggregate in the aqueous intestinal environment to form micelles that solubilise the digestion products of triglycerides (fatty acids and monoglycerides), promoting absorption. Conjugated BAs are actively absorbed by enterocytes of the terminal ileum via the apical sodium-dependent BA transporter (ASBT) and the basolateral organic solute transporter (Ostα/Ostβ). The small amounts of secreted BAs that escape ileal uptake 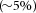 enter the colon, where bacterial enzymes deconjugate, oxidize and dehydroxylate the primary bile acids, forming the secondary BAs, which in humans include deoxycholic acid (DCA) and lithocholic acid (LCA). These transformations increase the hydrophobicity of secondary BAs, which are then passively absorbed across colonocytes. Absorbed BAs pass through the portal circulation back to the liver, where they may be reconjugated and stored in the gallbladder for re-use.
enter the colon, where bacterial enzymes deconjugate, oxidize and dehydroxylate the primary bile acids, forming the secondary BAs, which in humans include deoxycholic acid (DCA) and lithocholic acid (LCA). These transformations increase the hydrophobicity of secondary BAs, which are then passively absorbed across colonocytes. Absorbed BAs pass through the portal circulation back to the liver, where they may be reconjugated and stored in the gallbladder for re-use.
Figure 1. Synthesis, secretion and enterohepatic circulation of BAs in humans.
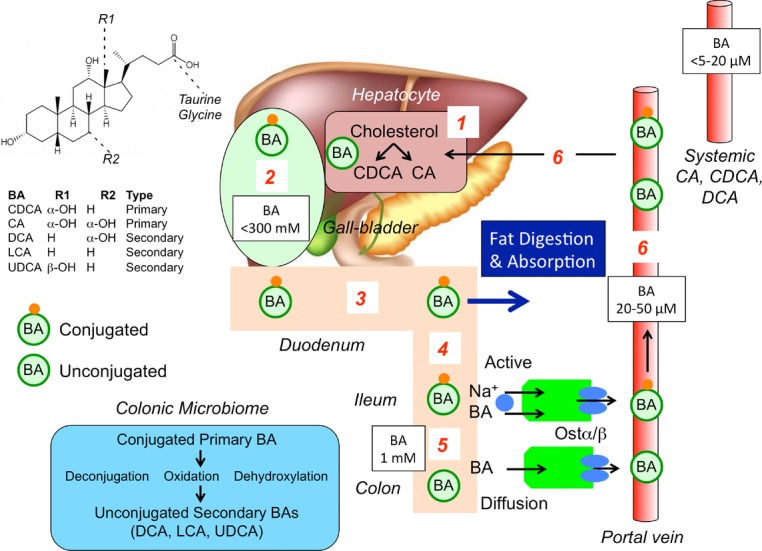
(1) Primary bile acids are synthesized in hepatocytes from cholesterol. (2) BAs are conjugated to glycine and taurine and are stored in the gallbladder at high concentrations. (3) After feeding, conjugated BAs are secreted in the intestine where they emulsify dietary fats and form mixed micelles that facilitate digestion and absorption of the products of triglyceride digestion. (4) Conjugated BAs are not passively absorbed in the small intestine, but are actively and efficiently absorbed in the terminal ileum by the apical sodium BA co-transporter at the apical membrane of enterocytes of the terminal ileum. (5) In the colon, bacteria deconjugate, oxidise and hydroxylatedehydroxylate conjugated primary bile acids to form secondary bile acids, which are passively absorbed. (6) Conjugated and unconjugated BAs enter to portal vein and recirculate to the liver for reuse Ursodeoxycholic acid (UDCA).
In view of the episodic nature of bile secretion and absorption, the concentrations of BAs in the intestine and circulation wax and wane during feeding and fasting (Angelin et al. 1982), reminiscent of the circulating levels of gut hormones. Indeed, BAs have hormone-like actions on multiple cells types. Nuclear receptors (e.g. farnesoid X receptor) mediate the genomic actions of BAs (reviewed in Lefebvre et al. 2009). TGR5 is a plasma membrane BA receptor that mediates the rapid, non-genomic effects of BAs (Lieu et al. 2014).
TGR5 is a G protein-coupled BA receptor
TGR5 is a seven transmembrane domain G protein-coupled receptor (GPCR) that couples to Gαs, leading to activation of adenylyl cyclase, formation of cAMP and stimulation of protein kinase A (PKA; Maruyama et al. 2002; Kawamata et al. 2003). In Chinese Hamster Ovary CHO-TGR5 cells, BAs stimulate cAMP production with an order of potency of taurolithocholic acid (TLCA) > LCA ≫ DCA > CDCA > CA (Kawamata et al. 2003). TGR5 also transactivates the epidermal growth factor receptor, which activates extracellular signal regulated kinases (Yasuda et al. 2007; Cao et al. 2013), and inhibits nuclear factor kappa-light-chain-enhancer of activated β cells NF-κB-mediated inflammatory signalling (Wang et al. 2011). In addition to generating cAMP, TGR5 activation in brain neurons and astrocytes can also increase [Ca2+]i, although whether this involves mobilization of intracellular calcium ions or activation of a calcium channel in the plasma membrane is unclear (Keitel et al. 2010).
An α-helical domain in the C-terminus facilitates TGR5 sorting to the plasma membrane and interaction with BAs in the extracellular fluid (Spomer et al. 2014). However, in contrast to most GPCRs, which desensitize and internalize after binding agonists, activated TGR5 remains at the plasma membrane where it can transmit sustained signals (Jensen et al. 2013). This unusual lack of desensitization may have implications for BA signalling and the therapeutic use of TGR5 agonists.
Neuro-humoral signalling by BAs and TGR5 in the gastrointestinal tract
BAs have many patho-physiological effects in the gastrointestinal tract (Fig. 2). They regulate hormone secretion, motility, electrolyte and fluid transport, formation and storage of bile, sensory transduction and inflammation. Some of these actions depend on TGR5 and others occur by unknown mechanisms. TGR5 is present in epithelial cells of the intestine and gallbladder, where the high concentrations of luminal BAs may activate the receptor at apical membranes. However, TGR5 is also expressed at the basolateral membrane of certain epithelial cells, and by neurons, smooth muscle cells and immune cells (Fig. 3). Although the permeability of the intestinal epithelium to BAs varies between regions and for particular BAs, the secondary BAs (LCA and DCA) are lipophilic and readily cross membranes, and circulating levels of BAs wax and wane during feeding and fasting (Angelin et al. 1982). Thus, it is probable that absorbed BAs can regulate multiple cells within the gut wall and beyond. The levels of BAs in the intestinal lumen, circulation and tissues also vary due to disease and therapy, which can lead to TGR5-dependent pathology.
Figure 2. Potential functions of TGR5 in the digestive system.
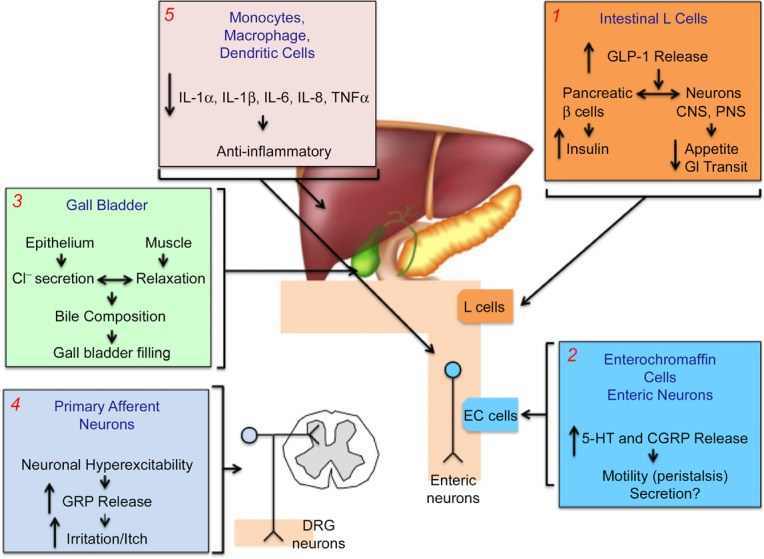
(1) Luminal BAs activate TGR5 on L cells to stimulate release of GLP-1, which promotes insulin secretion and inhibits food intake and gastric emptying, all of which lower blood glucose. (2) In the colon, BAs activate TGR5 on EC cells to stimulate release of 5–HT and on enteric neurons to stimulate release of CGRP. 5–HT and CGRP promote peristalsis. (3) In the gallbladder, activation of TGR5 on epithelial cells stimulates chloride secretion and activation of TGR5 on myocytes inhibits contractility. (4) In the skin and possibly in the intestine, BAs activate TGR5 on primary sensory neurons to induce hyperexcitability and release of GRP, a transmitter of itch and possibly irritant sensations. (5) BAs can activate TGR5 on immune cells of the liver and colon to suppress the release of proinflammatory cytokines and thereby dampen inflammation.
Figure 3. Neuro-humoral signalling of BAs and TGR5 in the gastrointestinal wall.
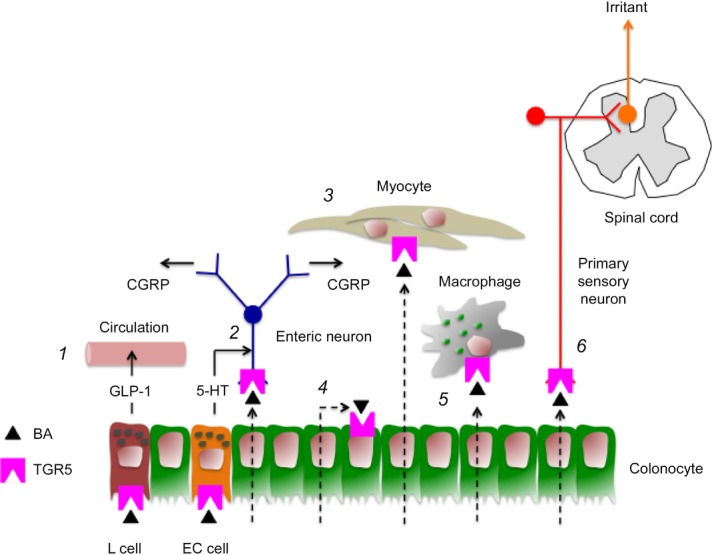
(1) In the small intestine, luminal BAs can activate TGR5 on L cells to induce secretion of the hormone GLP-1. (2) In the colon, luminal BAs can activate TGR5 on EC cells to stimulate secretion of 5–HT, which activates 5–HT4 receptors on intrinsic primary afferent neurons, which release CGRP, a mediator of the ascending and descending components of the peristaltic reflex. Absorbed BAs may directly activate TGR5 on enteric neurons to induce release of CGRP. (3) TGR5 is expressed by gastric myocytes, where activation by absorbed BAs can inhibit contractility. (4) Absorbed BAs may activate TGR5 on colonocytes to inhibit chloride secretion. (5) Absorbed BAs activate TGR5 on colonic macrophages to suppress secretion of tumour necrosis factor α and dampen inflammation. (6) By analogy to the skin, absorbed BAs may activate TGR5 on primary sensory neurons that transmit irritant signals to the spinal cord.
Hormone secretion
Glucagon-like peptide-1 (GLP-1) is a hormone from enteroendocrine L cells of the small intestine that lowers blood glucose by stimulating secretion of insulin through suppression of food intake and gastric emptying. BAs stimulate TGR5-dependent release of GLP-1 from the mouse enteroendocrine cell line STC-1 (Katsuma et al. 2005), and BA-evoked activation of TGR5 in the intestine stimulates GLP-1 release and thereby improves glucose homeostasis in obese mice (Thomas et al. 2009; Fig. 3). This mechanism may account for the beneficial effects of anionic exchange resins on glycaemic control in type 2 diabetes mellitus. By sequestering BAs in the lumen of the small intestine, resins suppress BA and lipid absorption, promote cholesterol conversion to BAs, and are therapeutically valuable cholesterol-lowering drugs. These drugs improve hyperglycaemia in obese mice by TGR5-dependent mechanisms (Harach et al. 2012; Potthoff et al. 2013). When fed to obese mice, BA sequestrants improve glucose homeostasis by a mechanism that involves TGR5-dependent upregulation in the colon of pre-proglucagon expression and GLP-1 release (Harach et al. 2012). Although there are elevated levels of total BAs in the faeces of mice treated with sequestrants, whether treatment with sequestrants also increases colonic levels of unbound BAs that are able to stimulate GLP-1 release from colonic L cells remains to be determined. The resins also suppress hepatic glucose production in obese mice by TGR5- and GLP-1-dependent inhibition of hepatic glycogenolysis (Potthoff et al. 2013). However, TGR5 agonists can also activate TGR5 on pancreatic β cells to stimulate insulin secretion (Kumar et al. 2012). These findings provided a rationale for the use of TGR5 agonists to treat type 2 diabetes.
Motility
BAs have regional effects on gastrointestinal motility. When administered by gavage, BAs inhibit gastric emptying and small intestinal transit (Poole et al. 2010). These effects may be mediated by activation of TGR5 on enteroendocrine cells, enteric neurons or myocytes (Fig. 3). BA-evoked release of GLP-1 could slow gastric emptying and transit, which remains to be investigated. TGR5 is expressed by inhibitory motor neurons of the myenteric plexus, and BAs that activate TGR5, such as DCA, inhibit spontaneous contractions of longitudinal muscle in isolated segments of mouse ileum by a neurogenic and nitrergic mechanism (Poole et al. 2010). TGR5 is also expressed by gastric myocytes, where activation causes relaxation through inhibition of the RhoA/Rho kinase pathway (Rajagopal et al. 2013).
Conversely, when applied to the mucosal surface of colonic segments at physiological concentrations, DCA and LCA stimulate peristalsis in wild-type but not Tgr5–/– mice (Alemi et al. 2013b). BAs activate TGR5 on enterochromaffin (EC) cells and intrinsic primary afferent neurons to stimulate release of the peristaltic transmitters 5-hydroxytryptamine (5-HT) and calcitonin gene-related peptide (CGRP) (Fig. 3). Thus, luminal BAs may activate TGR5 on EC cells to induce release of 5-HT, which activates 5-HT4 receptors on intrinsic primary afferent neurons to stimulate release of CGRP. Once absorbed, BAs may also directly stimulate CGRP release. CGRP stimulates ascending and descending interneurons that transmit the peristaltic reflex. The importance of BA-evoked and TGR5-dependent colonic peristalsis is underscored by the finding that overall gastrointestinal transit is impaired in Tgr5–/– mice, which are constipated, whereas Tgr5-transgenic mice show accelerated colonic transit (Alemi et al. 2013b).
Increased colonic delivery of BAs, which can occur after cholecystectomy due to continuous bile secretion and after small bowel inflammation or resection due to diminished BA absorption, can cause diarrhoea, perhaps by overactivation of TGR5. Decreased delivery of BAs, which can occur during cholestatic disease due to decreased bile secretion, can cause constipation, which may depend on depressed TGR5 activity. These findings have therapeutic implications. BAs have been ingested for millennia to treat constipation (Thorner, 1955), and inhibitors of ileal BA transporters relieve constipation by increasing colonic delivery of BAs (Camilleri, 2012). TGR5 agonists may also be treatments for constipation, whereas antagonists could be therapies for diarrhoea. Moreover, genetic variations in TGR5 may contribute to altered small bowel transit (Camilleri et al. 2011).
Electrolyte and fluid transport
BAs can both stimulate and inhibit electrolyte and fluid secretion in the colon. Whereas acute exposure of T84 colonocytes to high concentrations of DCA stimulates chloride secretion, which may be secondary to cell injury, chronic exposure to low concentrations of BAs inhibits the actions of calcium- and cAMP-dependent secretagogues (Keating et al. 2009). Although the mechanisms of these effects are uncertain, TGR5 has been implicated as a mediator of the inhibitory actions of BAs in the rat colon. TGR5 is highly expressed in colonic crypts, and the TGR5 agonists inhibit basal and carbachol-stimulated chloride secretion from isolated, nerve-free segments of rat colon (Ward et al. 2013). Although the physiological relevance of the anti-secretory actions of BAs remain to be defined, this mechanism may promote fluid absorption and thereby restrict fluid loss after excess colonic delivery of BAs. Since TGR5 is also expressed by submucosal neurons (Poole et al. 2010), further studies are required to determine whether BAs can also regulate transport by a neurogenic mechanism. However, the bile salt sodium deoxycholate stimulates secretion from the rat small intestine by a neuronal mechanism (Karlström et al. 1986), suggesting that TGR5 expressed by submucosal neurons may also contribute to the effects of BAs on electrolyte and fluid secretion.
Bile formation and storage
Since BAs are present in high concentrations in bile, and TGR5 is abundant in gallbladder epithelium and muscle, it is not surprising that TGR5 controls bile formation and storage. BAs stimulate TGR5- and cAMP/PKA-dependent activation of the cystic fibrosis transmembrane conductance regulator (CFTR) channel in gallbladder epithelial cells, leading to chloride secretion (Keitel et al. 2009). Hydrophobic bile salts stimulate TGR5-, cAMP/PKA-dependent activation of ATP-sensitive potassium channels in gallbladder myocytes, leading to hyperpolarization and decreased contractility (Lavoie et al. 2010). The importance of TGR5-induced secretion and relaxation of the gallbladder is highlighted by the finding that BAs and TGR5-selective agonists increase gallbladder volume in wild-type but not Tgr5–/– mice (Li et al. 2011). The existence of this intrinsic mechanism involving TGR5 that regulates gallbladder filling has implications for the side-effects of TGR5 agonists that are under development for the treatment of metabolic diseases.
Sensory transduction
Patients with cholestatic disease have elevated circulating and tissue levels of BAs and sometimes exhibit sensory abnormalities, which include an intractable pruritus. TGR5 mediates the pruritogenic actions of BAs in mice, and may thereby contribute to sensory abnormalities in patients with cholestatic disease (Alemi et al. 2013a). TGR5 is expressed in a subpopulation of small-diameter neurons of dorsal root ganglia, and BAs cause hyperexcitability of these neurons and evoke scratching by TGR5-dependent mechanisms (Alemi et al. 2013a). Itch is a protective mechanism that results in scratching, which removes potentially damaging irritants from the skin. The sensation of itch derives only after irritant stimulation to the skin. Given that the gut lumen is replete with irritants, including BAs that can excite sensory nerves, it is probable that similar irritant reflexes exist within the gut, a possibility that deserves further investigation.
Inflammation
TGR5 has been implicated in both induction and suppression of inflammation. The reflux of bile into the pancreatic duct is a potential cause of acute pancreatitis, and TGR5 has been implicated as a mediator of BA-induced pancreatitis (Perides et al. 2010). TGR5 is localized to the apical pole of acinar cells, and the retrograde infusion of taurolithocholic acid-3-sulfate into the pancreatic duct causes pancreatitis in wild-type but not Tgr5–/– mice. Isolated acinar cells lacking TGR5 are protected from the actions of BAs on pathological calcium transients, intracellular activation of zymogens, and injury. Although these findings implicate TGR5 on acinar cells as a mediator of pancreatitis after bile reflux, the physiological function of TGR5 in the exocrine pancreas is unknown.
Other studies point to an immunosuppressive, anti-inflammatory function of TGR5. TGR5 is expressed by peripheral blood monocytes, differentiated macrophages and dendritic cells, and TGR5 activation inhibits cytokine generation by macrophages (Kawamata et al. 2003; Yoneno et al. 2013; Fig. 3). TGR5 is upregulated in intestinal macrophages from patients with Crohn's disease, where activation inhibits the production of tumour necrosis factor-α (Kawamata et al. 2003; Yoneno et al. 2013). The TGR5 gene on chromosome 2q35 is close to a genetic variant associated with ulcerative colitis and primary sclerosing cholangitis, a chronic inflammatory condition of the bile duct, and several loss-of-function TGR5 mutations have been identified in primary sclerosing cholangitis (Hov et al. 2011). Further studies are required to determine the contribution of TGR5 to inflammatory diseases of the gastrointestinal system. However, TGR5 inhibits NF-κB-mediated inflammatory signalling and Tgr5–/– mice exhibit enhanced lipopolysaccharide-induced hepatitis, indicating the importance of this anti-inflammatory pathway (Pols et al. 2011; Wang et al. 2011).
Conclusions
Although the effects of BAs on the gastrointestinal tract have been recognized for millennia, only recently have the receptors that mediate these effects been described. Of these, TGR5 has been identified as a mediator of many of the rapid and non-genomic patho-physiological effects of BAs. Physiological roles of TGR5 in the digestive system include stimulation of GLP-1 secretion and control of blood glucose, control of transit and electrolyte and fluid transport in the colon, and regulation of the formation and storage of bile. TGR5 may also participate in irritant sensory reflexes in the digestive system and control inflammation. These findings have provided an impetus for the development of TGR5 agonists to treat disorders of metabolism, inflammation and motility. However, given the role of TGR5 in gallbladder function, itch and proliferation, TGR5 agonists may have detrimental side-effects, and in some cases antagonists may be useful therapies. Further understanding of the functions of TGR5 in the digestive system requires TGR5 knockout and transgenic mice and studies with TGR5-selective agonists, and a deeper understanding of the process of TGR5 signalling and regulation. Furthermore, it is possible that molecules that are structurally related to BAs are also agonists of TGR5 in the gut. The observation that neurosteroids can activate TGR5 (Keitel et al. 2010) suggests that studies of neurosteroid signalling in the digestive system are warranted.
Glossary
- BA
bile acid
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CGRP
calcitonin gene-related peptide
- DCA
deoxycholic acid
- EC cell
enterochromaffin cell
- GLP-1
glucagon-like peptide-1
- GPCR
G protein-coupled receptor
- GRP
gastrin releasing peptide
- LCA
lithocholic acid
Additional information
Competing interests
None declared.
Funding
This work is supported by NHMRC 63303, 1049682, 1031886 and Monash University.
References
- Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013a;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013b;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Pharmacology of the new treatments for lower gastrointestinal motility disorders and irritable bowel syndrome. Clin Pharmacol Ther. 2012;91:44–59. doi: 10.1038/clpt.2011.261. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Vazquez-Roque MI, Carlson P, Burton D, Wong BS, Zinsmeister AR. Association of bile acid receptor TGR5 variation and transit in health and lower functional gastrointestinal disorders. Neurogastroenterol Motil. 2011;23:995–999. doi: 10.1111/j.1365-2982.2011.01772.x. e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Tian W, Hong J, Li D, Tavares R, Noble L, Moss SF, Resnick MB. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2013;304:G322–G327. doi: 10.1152/ajpgi.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach T, Pols TW, Nomura M, Maida A, Watanabe M, Auwerx J, Schoonjans K. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep. 2012;2:430. doi: 10.1038/srep00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hov JR, Keitel V, Schrumpf E, Haussinger D, Karlsen TH. TGR5 sequence variation in primary sclerosing cholangitis. Dig Dis. 2011;29:78–84. doi: 10.1159/000324138. [DOI] [PubMed] [Google Scholar]
- Jensen DD, Godfrey CB, Niklas C, Canals M, Kocan M, Poole DP, Murphy JE, Alemi F, Cottrell GS, Korbmacher C, Lambert NA, Bunnett NW, Corvera CU. The bile acid receptor TGR5 does not interact with β-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. J Biol Chem. 2013;288:22942–22960. doi: 10.1074/jbc.M113.455774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlström L, Cassuto J, Jodal M, Lundgren O. Involvement of the enteric nervous system in the intestinal secretion induced by sodium deoxycholate and sodium ricinoleate. Scand J Gastroenterol. 1986;21:331–340. doi: 10.3109/00365528609003083. [DOI] [PubMed] [Google Scholar]
- Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- Keating N, Mroz MS, Scharl MM, Marsh C, Ferguson G, Hofmann AF, Keely SJ. Physiological concentrations of bile acids down-regulate agonist induced secretion in colonic epithelial cells. J Cell Mol Med. 2009;13:2293–2303. doi: 10.1111/j.1582-4934.2009.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- Kumar DP, Rajagopal S, Mahavadi S, Mirshahi F, Grider JR, Murthy KS, Sanyal AJ. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427:600–605. doi: 10.1016/j.bbrc.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Balemba OB, Godfrey C, Watson CA, Vassileva G, Corvera CU, Nelson MT, Mawe GM. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol. 2010;588:3295–3305. doi: 10.1113/jphysiol.2010.192146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, Kliewer SA, Mangelsdorf DJ. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25:1066–1071. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu T, Jayaweera G, Bunnett NW. Gpba: A G protein-coupled receptor for bile acids and an emerging therapeutic target for disorders of digestion and sensation. Br J Pharmacol. 2014;171:1156–1166. doi: 10.1111/bph.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138:715–725. doi: 10.1053/j.gastro.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. e227–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, Mangelsdorf DJ, Kliewer SA, Burgess SC. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G371–G380. doi: 10.1152/ajpgi.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Kumar DP, Mahavadi S, Bhattacharya S, Zhou R, Corvera CU, Bunnett NW, Grider JR, Murthy KS. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am J Physiol Gastrointest Liver Physiol. 2013;304:G527–G535. doi: 10.1152/ajpgi.00388.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spomer L, Gertzen CG, Schmitz B, Häussinger D, Gohlke H, Keitel V. A membrane-proximal, C-terminal α-helix is required for plasma membrane localization and function of the G protein-coupled receptor (GPCR) TGR5. J Biol Chem. 2014;289:3689–3702. doi: 10.1074/jbc.M113.502344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5–connecting nutrition and metabolism. Thyroid. 2008;18:167–174. doi: 10.1089/thy.2007.0255. [DOI] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner RS. The effect of exclusion of the bile upon gastrointestinal motility. Am J Roentgenol Radium Ther Nucl Med. 1955;74:1096–1122. [PubMed] [Google Scholar]
- Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil. 2013;25:708–711. doi: 10.1111/nmo.12148. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354:154–159. doi: 10.1016/j.bbrc.2006.12.168. [DOI] [PubMed] [Google Scholar]
- Yoneno K, Hisamatsu T, Shimamura K, Kamada N, Ichikawa R, Kitazume MT, Mori M, Uo M, Namikawa Y, Matsuoka K, Sato T, Koganei K, Sugita A, Kanai T, Hibi T. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease. Immunology. 2013;139:19–29. doi: 10.1111/imm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]


