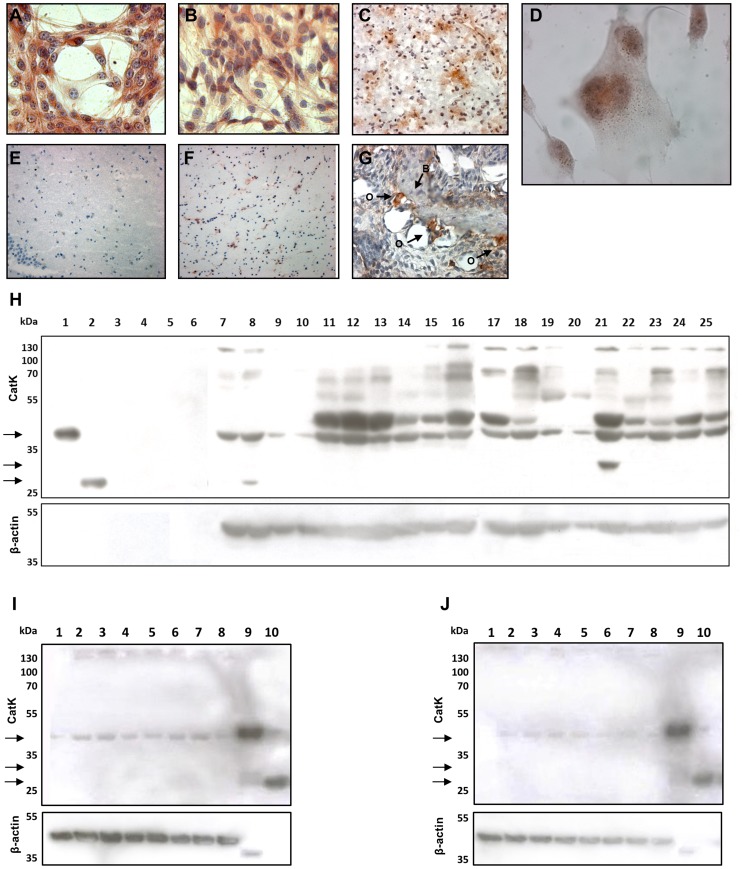
Figure 6. Immunohistochemstry and immunocytochemstry and Western blot analysis of cathepsin K in GBM cells and tissues.
ICC staining of CatK in U87-MG (A) and U373 (B, D) GBM cells. At high magnification (1000×), a granular pattern of the staining was observed in U373 cells (D), which corresponds to perinuclear endo-lysosomal-like localization of CatK. Strong IHC staining of CatK in GBM tissue (C), and weak staining in control brain tissue (non-malignant brain) (E, F). Mouse jaw with bone tissue (B) with osteoclasts at bone edges (O) was used as positive control for CatK staining (G). Magnifications: A, B, G - 400×; D - 1000×; C, E, F - 200×. (H) Western blot of GBM cells (lanes 7–10) and their conditioned media (CM; lanes 3–6), GBM tissue (lanes 17–25) and non-malignant brain tissue (lanes 11–16) showing positivity for pro-CatK (39 kDa). The active form of the enzyme (27 kDa) was detected only in a small amount in one passage of U87-MG cells (lane 8). At 30 kDa, an intermediate form of CatK was observed (lane 21). Recombinant proform (lane 1) and active CatK (lane 2) were used as positive control. As loading control β-actin was used. Legend: 1 – recombinant pro-CatK, 2 – recombinant active CatK, 3 – U87p38 CM, 4 – U87p39 CM, 5 – U373p41 CM, 6 – U373p42 CM, 7 – U87p45, 8 – U87p48, 9 – U373p45, 10 – U373p48, 11–16 – different non-malignant brain samples, 17–25 – different GBM tissue samples. Please note that Western blotting image does not allow for direct quantitative comparison of CatK expression in control and tumor samples due to variable protein amounts loaded. (I and J) Additional western blot experiments using cell lines U87-MG (I) and U373 (J) and different protease inhibitors. No active form of cathepsin K was observed in any of the conditions tested but in all cases pro-cathepsin K was present. Legend: 1 – without any inhibitor, 2 – 5 µM E-64, 3 – 5 µM CA074, 4 – 5 µM CLIK148, 5 – 5 µM pepstatin A, 6 – 1 mM PMSF, 7 – 20 mM EDTA, 8 – combination of all inhibitors, 9 – control: recombinant pro-CatK, 10 – control: recombinant active CatK.