FIGURE 2:
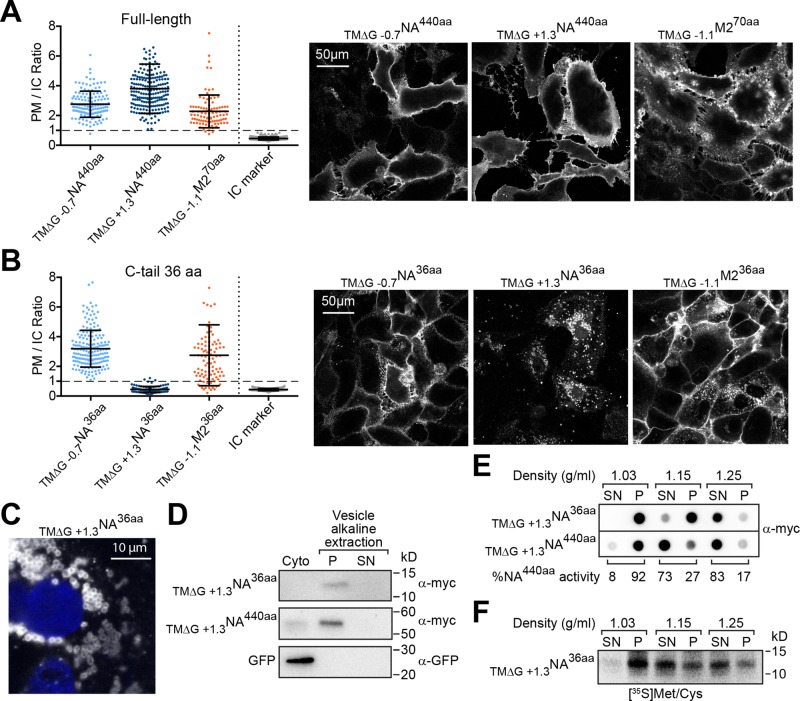
Marginally hydrophobic NA TMDs with a short C-tail mislocalize in cells. (A) Cell PM/IC ratios for the full-length Nin-Cout membrane protein NA440aa with a hydrophobic (ΔGapp = −0.7 kcal/mol) and a marginally hydrophobic (ΔGapp = +1.3 kcal/mol) TMD, as well as the Nout-Cin membrane protein M270aa with a hydrophobic (ΔGapp = −1.1 kcal/mol) TMD and the intracellular (IC) marker. Right, representative cell section images. (B) PM/IC ratios from cells expressing these membrane proteins with a C-tail truncated to 36 aa. Right, representative images. (C) Higher-magnification image showing localization of TM∆G +1.3NA36aa (white) in “ring-like” vesicle structures near the nucleus (blue). (D) Vesicles from cells transfected with the indicated constructs were separated from the cytoplasm (Cyto) and subjected to an alkaline extraction to separate integral (P) from peripheral (SN) membrane proteins. Representative immunoblots with GFP as the soluble cytoplasm control. (E) Vesicle and cytoplasmic fractions from transfected cells were analyzed by flotation on sucrose cushions of different density. The dot blot shows the intensity of TM∆G +1.3NA36aa and TM∆G +1.3NA440aa that floated (SN) or pelleted (P) at each density, and the enzymatic activity distribution for TM∆G +1.3NA440aa provides an added control. (F) Similar analysis as in E, except that TM∆G +1.3NA36aa was radiolabeled for 30 min and isolated after the separation using Ni-Sepharose before resolution by SDS–PAGE.