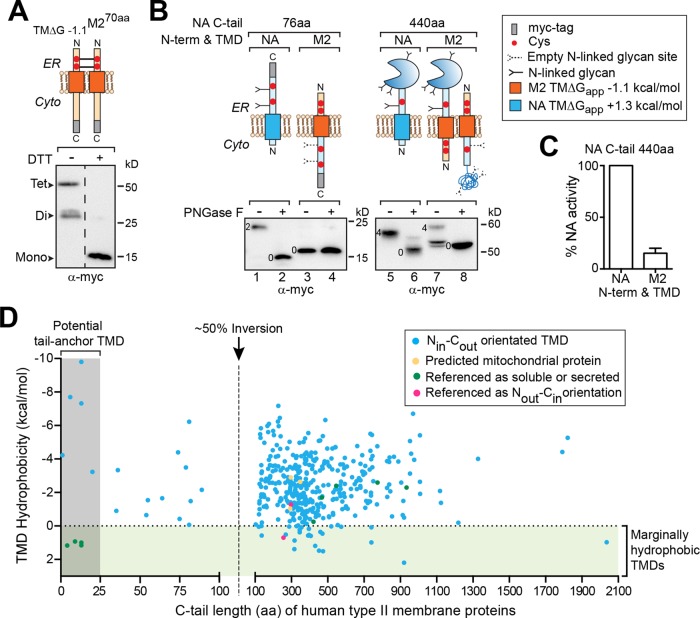
FIGURE 8:
An elongated C-tail can invert the Nout-Cin M2 TMD and is associated with marginally hydrophobic human Nin-Cout (type II) TMDs. (A) Immunoblot of reduced and nonreduced cell lysates showing the Nout-Cin orientation of M2 based on the intermolecular disulfide bonds formed by its N-terminal Cys residues in the ER lumen. (B) Immunoblots of untreated and PNGase F–treated lysates showing the glycosylation patterns for TMΔG+1.3NA76aa, M2 with the 76-aa NA C-tail, full-length NA (TMΔG+1.3NA440aa), and M2 with the full-length 440-aa NA C-tail with the enzymatic domain. The number of N-linked glycans for each species is indicated. (C) Enzymatic activity was used to confirm M2 TMD inversion, as NA only folds within the ER lumen. The activity rate of M2 with the full-length 440-aa NA C-tail was calculated in comparison to lysates from cells expressing full-length NA as described in da Silva et al. (2013). (D) TMD hydrophobicity for the annotated type II human membrane proteins with respect to the length of the C-terminus after their TMD (C-tail). Dashed line at 100 aa corresponds to the ∼50% inversion point for newly synthesized NA with a marginally hydrophobic TMD. Regions covering the marginally hydrophobic TMDs and potential tail-anchored pathway substrates are highlighted. Protein sequences were obtained from Uniprot and analyzed using MPEx. The raw data and references are given in Supplemental Table S1.