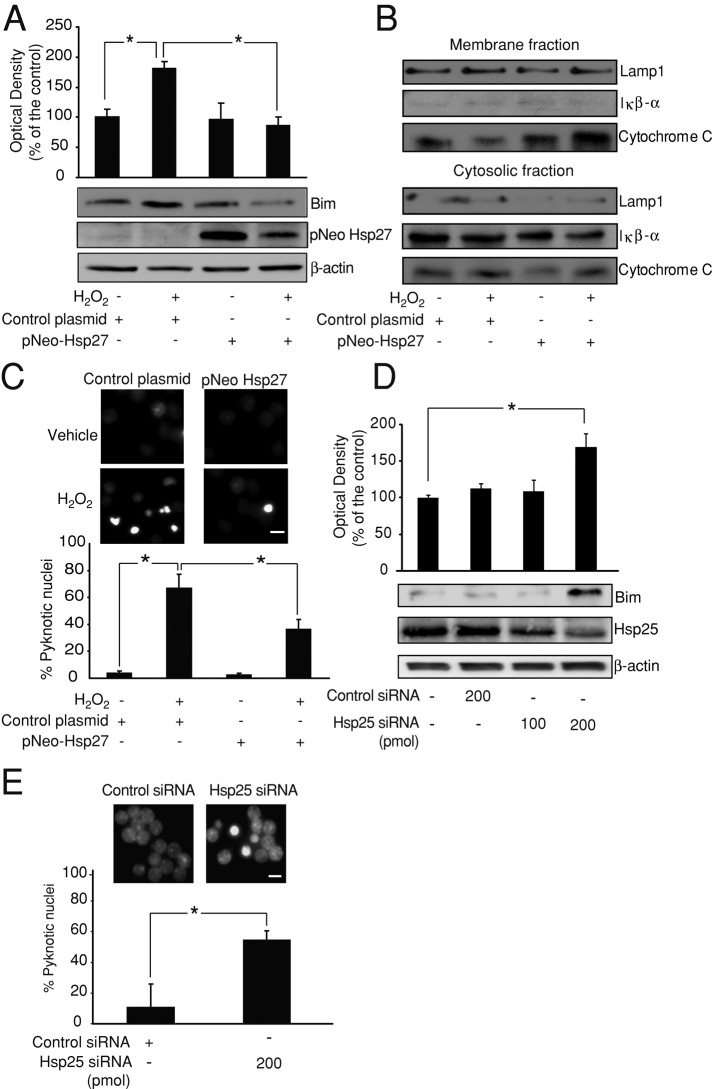
FIGURE 2:
Hsp27 regulates Bim protein levels and oxidative stress–induced cell death. (A) CGNs were transfected with pNEO-Hsp27 or a control construct before H2O2 (37.5 μM) addition. pNEO-Hsp27 neurons displayed significantly lower Bim protein levels than did control neurons after H2O2 treatment (*p < 0.05; n = 3). β-Actin served as loading control. (B) At 4 h after H2O2 (37.5 μM) treatment, pNEO-Hsp27 neurons displayed lower cytosolic cytochrome C protein levels than in control neurons. Mitochondrial membrane levels of cytochrome C were also higher in pNEO-Hsp27 neurons than in control neurons. IκB-α protein served as marker of cytosolic fraction and LAMP1 as marker of the membrane fraction, including mitochondrial membranes. (C) CGNs transfected with pNEO-Hsp27 or a control construct were treated with H2O2 (37.5 μM) or sham conditions and live-stained with Hoechst 4–6 h later. pNEO-Hsp27 neurons showed a significantly lower percentage of cells with pyknotic nuclei than control neurons after H2O2 addition (*p < 0.05; n = 3). Bar, 2.5 μm. (D) CGNs were electroporated with Hsp25 siRNA (rodent homologue of Hsp27; 100/200 pmol) or control siRNA (200 pmol), and Bim and Hsp25 protein levels were analyzed after 72 h by Western blot. Hsp25 levels were depleted in Hsp25 siRNA (200 pmol) neurons, which also displayed significantly higher levels of Bim than control siRNA neurons (*p < 0.05; n = 3). β-Actin served as loading control. (E) CGNs electroporated with Hsp25 siRNA or control siRNA were live-stained with Hoechst. Hsp25 siRNA neurons presented a significantly higher percentage of cells with pyknotic nuclei than control neurons (*p < 0.05; n = 3). Bar, 2.5 μm.